Reperfusion therapy is generally recommended in acute high-risk pulmonary embolism (HR-PE), but several population-based studies report that it is underused. Data on epidemiology, management and outcomes of HR-PE in Portugal are scarce.
ObjectiveTo determine the reperfusion rate in HR-PE patients, the reasons for non-reperfusion, and how it influences outcomes.
MethodsIn this retrospective cohort study of consecutive HR-PE patients admitted to a thromboembolic disease referral center between 2008 and 2018, independent predictors for non-reperfusion were assessed by multivariate logistic regression. PE-related mortality and long-term MACE (cardiovascular mortality, PE recurrence and chronic thromboembolic disease) were calculated according to the Kaplan–Meier method. Differences stratified by reperfusion were assessed using the log-rank test.
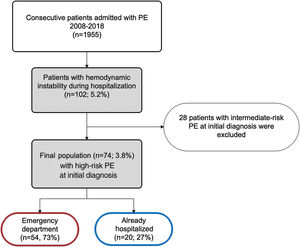
ResultsOf 1955 acute PE patients, 3.8% presented with hemodynamic instability. The overall reperfusion rate was 50%: 35 patients underwent systemic thrombolysis, one received first-line percutaneous embolectomy and one rescue endovascular treatment. Independent predictors of non-reperfusion were: age, with >75 years representing 12 times the risk of non-treatment (OR 11.9, 95% CI 2.7–52.3, p=0.001); absolute contraindication for thrombolysis (31.1%), with recent major surgery and central nervous system disease as the most common reasons (OR 16.7, 95% CI 3.2–87.0, p<0.001); and being hospitalized (OR 7.7, 95% CI 1.4–42.9, p=0.020). At a mean follow-up of 2.5±3.3 years, the survival rate was 33.8%. Although not reaching statistical significance for hospital mortality, mortality in the reperfusion group was significantly lower at 30 days, 12 months and during follow-up (relative risk reduction of death of 64% at 12 months, p=0.013). Similar results were found for MACE.
ConclusionsIn this population, the recommended reperfusion therapy was performed in only 50% of patients, with advanced age and absolute contraindications to fibrinolysis being the main predictors of non-reperfusion. In this study, thrombolysis underuse was associated with a significant increase in short- and long-term mortality and events.
A reperfusão é geralmente o tratamento recomendado no tromboembolismo pulmonar de alto risco (TEP-AR), embora vários estudos populacionais reportem a sua subutilização. Em Portugal, não existem dados sobre incidência, tratamento e eventos nesta população.
ObjetivosEstimar a taxa de reperfusão na população com TEP-AR, os motivos de não reperfusão (NR) e o seu impacto nos resultados clínicos destes doentes (dts).
MétodosCoorte retrospetiva de dts consecutivos com TEP-AR num centro de referência (2008 a 2018). Os preditores independentes de NR foram estudados através de regressão logística multivariada. As incidências de mortalidade relacionada com TEP e de MACE no seguimento (morte cardiovascular, recorrência de TEP e hipertensão pulmonar tromboembólica crónica) foram calculadas pelo método de Kaplan-Meier. As diferenças entre grupos de reperfusão foram estratificadas pelo teste log-rank.
ResultadosDe 1955 dts com TEP agudo, 3,8% apresentaram-se hemodinamicamente instáveis. A taxa de reperfusão total foi 50% - 35 dts com trombólise sistémica, 1 dt com embolectomia percutânea primária e 1 dt com tratamento endovascular de recurso. Os preditores independentes de NR foram: idade, ter > 75 anos associou-se a 12 vezes mais risco de NR (OR 11,9, 95%CI 2,7-52,3, p=0,001); contraindicações absolutas para trombólise (31,1%), sendo a cirurgia major recente e a doença do sistema nervoso central as causas mais comuns (OR 16,7, IC95% 3,2-87,0, p<0,001); estar hospitalizado (OR 7,7, 95%CI 1,4-42,9, p=0,020). Com um seguimento médio de 2,5±3,3 anos, a sobrevida foi de 33,8%. Apesar de o impacto na mortalidade intra-hospitalar não ter atingido significado estatisticamente significativo. O grupo da reperfusão mostrou uma melhoria significativa da mortalidade aos 30 dias, 12 meses e no seguimento (com uma redução de risco relativo de 64% na mortalidade a 12 meses, p=0,013). Os resultados na redução de MACE foram semelhantes.
ConclusõesNa população analisada, a terapêutica recomendada de reperfusão foi registada em apenas 50% dos doentes, sendo a idade avançada e a presença de contraindicações absolutas para fibrinólise os principais preditores de não tratamento. Neste estudo, a subutilização de trombólise associou-se a um aumento significativo de mortalidade e eventos cardiovasculares a curto e longo prazo.
Acute pulmonary embolism (PE) is a common disease (39–115/100000 population).1 In Portugal, its incidence has been estimated at 35/100000.2 It is one of the leading causes of cardiovascular death worldwide, with the highest mortality in patients who present or develop obstructive cardiogenic shock and hemodynamic instability (3–8%),1–5 i.e. those with high-risk PE (HR-PE).1
Early mortality (within three months) appears to be mostly related to right ventricular (RV) failure and PE recurrence. It has been estimated at 30%, 34.8% and 41.3% for death in-hospital,6,7 at 30 days,6 and at 90 days,6 respectively. Previous studies reveal very high long-term mortality in this patient group (71.4% at four years).4 Unlike short-term mortality, which is mainly related to the severity of PE presentation, long-term mortality is more likely to be linked to comorbidities than to the thromboembolic event itself.4,8
To rapidly restore pulmonary circulation and RV function, reperfusion therapy is the standard of care1 and significantly reduces early mortality in these patients: 49.9% vs. 28.6% during hospitalization8; 53.8% vs. 27.3% at 30 days6; and 61.5% vs. 33.3% at 90 days.6 The impact of reperfusion on long-term events is unknown.
Although reperfusion is the recommended strategy for the management for HR-PE patients, several population-based studies report that it is underused, with only 20–30% of patients receiving systemic fibrinolysis in real-world everyday practice.3,5,9 The most common reasons for non-reperfusion are older age, active cancer and recent surgery,5,8 but few of these represent absolute contraindications for the administration of systemic thrombolysis. In the presence of absolute or relative contraindications, alternatives to reperfusion such as surgical thrombectomy or percutaneous catheter-directed therapy (CDT) should be considered.1 There is an increasing tendency to use percutaneous strategies due to their positive impact on hospital mortality.10
In Portugal, the incidence of HR-PE and the rate of reperfusion in this patient group are unknown, as is its impact on short- and long-term survival.
ObjectivesThe main purposes of this study were to determine the reperfusion rate in HR-PE and the main reasons for non-reperfusion, and to analyze the impact of reperfusion on early and late major adverse cardiovascular events (MACE).
MethodsStudy populationThis retrospective cohort study in a tertiary care hospital included consecutive patients admitted with HR-PE over a 10-year period, using International Classification of Diseases (ICD)-9 codes for the period between January 1, 2008 and December 31, 2015 and ICD-10 for patients admitted between January 1, 2016 and December 31, 2018.
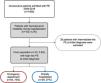
Patients who fulfilled HR-PE criteria according to the most recent European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines were included.1
Hemodynamically stable patients (low- or intermediate-risk PE) were excluded from the study, including those who developed hemodynamic instability after the initial PE diagnosis. Patients with a presumptive diagnosis (diagnosis not confirmed after imaging techniques or autopsy) were also excluded.
The incidence of HR-PE was calculated using the total population of 350000 that is estimated to be served by our hospital.
Data on demographic characteristics, comorbidities, clinical presentation, treatment, and clinical course were collected from clinical records. Based on these data, we calculated prognostic stratification scores that have been validated for less severe forms of PE (PESI [pulmonary embolism severity index] and sPESI [simplified PESI]).1 Data on events and mortality during follow-up were collected from hospital clinical records (emergency room or hospital admissions), as well as other hospital or primary care appointments available from a national electronic registry (Registo de Saúde Electrónico).
All patients were followed until the last available record or date of death.
The study protocol was approved by the ethics committee and was in accordance with the 1975 World Medical Association's Declaration of Helsinki. The corresponding authors assume full responsibility for the integrity of data and data analysis.
Study purpose and outcomesThe primary objective of this study was to estimate the reperfusion rate in the HR-PE population. Reperfusion was defined as systemic or catheter-directed fibrinolysis, surgical embolectomy, or percutaneous catheter-directed embolectomy.
Secondary objectives were to identify predictors of non-reperfusion and to determine the association of non-reperfusion with adverse events: in-hospital mortality, early mortality (at 30 days) and long-term mortality and MACE (at one year and during follow-up).
Thus, all-cause mortality was established as the primary endpoint. The secondary endpoint was defined as MACE – a composite of cardiovascular mortality, embolic recurrence and occurrence of chronic thromboembolic pulmonary hypertension (CTEPH) – during follow-up.
A safety endpoint was also defined as the occurrence of bleeding events, stratified by their severity according to the Bleeding Academic Research Consortium (BARC) classification.11
Study definitionsRespiratory exhaustion was defined as bradypnea (respiratory frequency <12 cycles/min [cpm]), marked polypnea (respiratory frequency >30 cpm), or need for endotracheal intubation.
Recurrent PE was defined as hospital admission for a new PE event or evidence of new symptomatic PE documented on computed tomography or perfusion defects on pulmonary scintigraphy, during a follow-up appointment.
CTEPH was defined according to the 2015 European guidelines on pulmonary hypertension (with a definite diagnosis of pulmonary hypertension based on measurements obtained during right heart catheterization).12
Statistical analysisContinuous variables were tested for normality and equality of variances using the Shapiro–Wilk test and the Levene test, respectively. Those that followed a normal distribution were presented as mean±standard deviation; those that followed a non-normal distribution were displayed as median±interquartile range. According to their distribution, groups were compared with the independent Student's t test or the Mann–Whitney test, respectively.
Categorical variables were presented as numbers and percentages and groups were compared using the chi-square test or Fisher's exact test, as appropriate.
Independent predictors of non-reperfusion were determined by multivariate logistic regression analysis. Variables inserted into the multivariate model were selected according to their significance in univariate testing (those with a p-value <0.1 and narrow 95% confidence intervals were included). The final model was built by selecting forward stepwise variables with entry criterion p=0.05 and exit criterion p=0.1. The model's goodness of fit was determined by calculating the Hosmer–Lemeshow statistic.
The cumulative incidence of events was estimated by the Kaplan–Meier method and their difference determined by the log-rank test. The benefit of reperfusion for long-term events was adjusted to the population characteristics using stepwise Cox regression analysis.
All reported p-values were two-sided and a p-value <0.05 was considered statistically significant.
The statistical analysis was performed with IBM SPSS Statistics 21.0 (IBM Corp, Armonk, NY, USA).
ResultsPopulation characteristicsBetween January 1, 2008 and December 31, 2018, 1955 patients were admitted to our hospital with a diagnosis of PE, resulting in an annual incidence of 58/100000 population. Of these, 74 patients (3.8%) were hemodynamically unstable at the time of the initial diagnosis, thus being considered as HR-PE and fulfilling the inclusion criteria for this study. Of the included patients, 73% (n=54) came from the emergency department (ED) and the other 27% (n=20) were already hospitalized for other reasons (Figure 1).
Patients’ demographic, clinical and laboratory characteristics are shown in Table 1. Mean age was 68±15 years and most patients were female (n=49, 66.2%). The prevalence of cardiovascular risk factors such as hypertension, dyslipidemia, diabetes and overweight/obesity was very high. This population showed a higher incidence of strongly predisposing PE risk factors as defined by the ESC/ERS guidelines1 (no factor 12.2% vs. weak factor 21.6% vs. moderate factor 28.4% vs. strong factor 37.8%). The mean PESI score was 201.3±38.4. Clinical presentation was characterized by severe hypotension and the presence of acidemia (mean pH 7.3±0.2), hyperlactacidemia (mean blood lactates 6.6±6.1 mmol/l) and kidney failure (mean blood creatinine 4.8±15.5 mg/ml). A significant proportion of the patients presented respiratory failure with hypoxemia (mean partial pressure of arterial oxygen/fraction of inspired oxygen [PaO2/FiO2] 200.4±163.3) and respiratory exhaustion was present in 25.7%.
Demographic, clinical and laboratory characteristics of the study population.
| Demographic characteristics | |
|---|---|
| Age, years (mean±SD) | 68.3±15.5 |
| Female | 49 (66.2%) |
| History of cardiovascular disease | |
| Hypertension, n (%) | 47 (63.5%) |
| Dyslipidemia, n (%) | 19 (25.7%) |
| Diabetes, n (%) | 20 (27%) |
| Overweight/obesity, n (%) | 20 (27%) |
| Active/previous smoking, n (%) | 14 (18.9%) |
| Coronary artery disease, n (%) | 8 (10.8%) |
| Heart failure, n (%) | 9 (12.2%) |
| Cerebrovascular disease, n (%) | 12 (16.2%) |
| Peripheral artery disease, n (%) | 1 (1.4%) |
| Other risk factors for VTE | |
| Hormonal contraception, n (%) | 4 (5.4%) |
| Previous VTE, n (%) | 9 (12.2%) |
| History of cancer, n (%) | 23 (31.1%) |
| Recent major surgery, n (%) | 15 (20.3%) |
| Immobility, n (%) | 22 (29.7%) |
| Prolonged travel, n (%) | 3 (4.1%) |
| VTE predisposing factors | |
| None, n (%) | 9 (12.2%) |
| Weak, n (%) | 16 (21.6%) |
| Moderate, n (%) | 21 (28.4%) |
| Strong, n (%) | 28 (37.8%) |
| Clinical presentation | |
| Vital signs | |
| SBP, mmHg (mean±SD) | 81.29±15.2 |
| HR, bpm (mean±SD) | 107.3±31.8 |
| Shock index (HR/SBP) (mean±SD) | 1.4±0.5 |
| PaO2/FiO2 (mean±SD) | 200.4±163.3 |
| Cardiac arrest presentation, n (%) | 8 (10.8%) |
| Respiratory exhaustion, n (%) | 19 (25.7%) |
| PESI score (mean±SD) | 201.3±38.4 |
| Laboratory tests | |
| pH (mean±SD) | 7.3±0.2 |
| Lactates, mmol/l (mean±SD) | 6.6±6.1 |
| Initial hs-troponin T, ng/ml (mean±SD) | 127.8±122.2 |
| Peak NT-proBNP, pg/ml (mean±SD) | 5768.2±8680.1 |
| Admission creatinine, mg/ml (mean±SD) | 4.8±15.5 |
bpm: beats per minute; HR: heart rate; hs: high-sensitivity; NT-proBNP: N-terminal pro-brain natriuretic peptide; PaO2/FiO2: partial pressure of arterial oxygen/fraction of inspired oxygen; PESI: pulmonary embolism severity index; SBP: systolic blood pressure; SD: standard deviation; VTE: venous thromboembolism.
Comparison of clinical severity between ED admission and hospitalized patients showed no statistically significant differences (Table 2).
Comparison of severity of clinical presentation between emergency department admissions and hospitalized patients.
| ED | Hospitalized | p | |
|---|---|---|---|
| Clinical presentation | |||
| Vital signs | |||
| SBP, mmHg, median (IQR) | 81.0 (70.0–90.0) | 84.0 (75.0–90.0) | 1.000 |
| HR, bpm, median (IQR) | 106.0 (91.5–125.0) | 120.0 (111.0–131.0) | 0.081 |
| Shock index (HR/SBP), median (IQR) | 1.27 (1.00–1.54) | 1.44 (1.18–1.66) | 0.375 |
| PaO2/FiO2, median (IQR) | 197.0 (94.5–270.0) | 180.0 (121.5–270.0) | 0.575 |
| Cardiac arrest, n (%) | 5 (9.3%) | 3 (15.0%) | 0.480 |
| Respiratory exhaustion, n (%) | 12 (22.6%) | 7 (36.8%) | 0.228 |
| PESI score (mean±SD) | 200.7 (173.5–230.0) | 202.7 (159.0–233.0) | 0.847 |
| Laboratory tests | |||
| pH, median (IQR) | 7.36 (7.25–7.43) | 7.32 (7.24–7.46) | 0.881 |
| Lactates, mmol/l, median (IQR) | 5.70 (2.40–8.60) | 5.48 (3.23–9.05) | 0.783 |
| Initial hs-troponin T, ng/ml, median (IQR) | 83.0 (46.0–192.0) | 54.0 (36.0–164.0) | 1.000 |
| NT-proBNP max, pg/ml, median (IQR) | 2447.0 (649.0–7682.8) | 1352.0 (341.0–9010.0) | 1.000 |
| Admission creatinine, mg/ml, median (IQR) | 1.3 (1.0–2.0) | 0.9 (0.7–2.3) | 0.216 |
bpm: beats per minute; ED: emergency department; HR: heart rate; hs: high-sensitivity; IQR: interquartile range; NT-proBNP: N-terminal pro-brain natriuretic peptide; PaO2/FiO2: partial pressure of arterial oxygen/fraction of inspired oxygen; PESI: pulmonary embolism severity index; SBP: systolic blood pressure; SD: standard deviation.
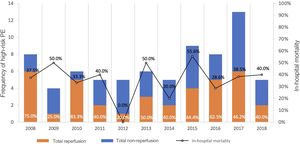
The total reperfusion rate was 50% (n=37): 35 patients underwent systemic fibrinolysis and two received CDT (one as a primary strategy and one as bailout after fibrinolysis failure). The reperfusion rate over the years and its impact on hospital mortality are presented in Figure 2. Of the 37 patients who did not receive reperfusion, 33 (89.2%) received full-dose parenteral anticoagulation: 32.4% (n=12) with unfractionated heparin and 56.8% (n=21) with low molecular weight heparin. Only four patients did not receive anticoagulation.
Analysis of variables associated with non-reperfusion showed that those that were statistically significant were older age (78±10 years vs. 61±19 years, p=0.02), previous history of coronary artery disease (87.5% vs. 45.5%, p=0.025), recent major surgery (86.7% vs. 40.7%, p=0.001) and the presence of absolute contraindications for fibrinolysis (87% vs. 45.9%, p<0.001). Some variables tended to be associated with non-reperfusion: previous cerebrovascular disease (75% vs. 45.2%, p=0.058), previous cancer (65.2% vs. 43.1%, p=0.079) and a higher mean PESI score (220.5±27.0 vs. 189.1±37.0 points, p=0.071). Moreover, although not reaching statistical significance, a higher rate of non-reperfusion was seen in patients with stronger predisposing factors (no factor 22.2% vs. weak factor 43.8% vs. moderate factor 52.4% vs. strong factor 60.7%, p=0.227).
Associated with a higher reperfusion rate were ED admission (80% vs. 38.9%, p=0.002), acidemia (pH 7.32±0.2 vs. 7.39±0.2, p=0.031) and hyperlactacidemia (6.85±1.1 vs. 3.59±2.1 mmol/l, p=0.022). Other variables including systolic blood pressure (SBP), shock index (ratio between heart rate and SBP) and PaO2/FiO2 were not associated with the type of therapy delivered.
After univariate analysis, variables that showed a statistically significant association with non-reperfusion were included in multivariate analysis (Table 3). Of the independent predictors for non-reperfusion, age over 75 years presented 12 times the risk of not receiving reperfusion therapy (reperfusion rate of 39.5% vs. 68.8%, p=0.004). Absolute contraindications for fibrinolysis were present in 31.1% of patients (Table 4) and were also independent predictors of non-reperfusion (reperfusion rate 18.5% vs. 72.0%, p<0.001): major surgery (13 patients) and central nervous system disease (eight patients) were the most frequent situations. This clinical scenario was more prevalent in previously hospitalized patients (60.9% vs. 11.8%, p<0.001), which in turn was also an independent predictor of non-reperfusion (39.3% vs. 64.9%, p=0.020).
Predictors of non-reperfusion after univariate and multivariate analysis.
| Predictors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | CI | p | OR | CI | p | |
| Age >75 years | 3.2 | 1.2–8.4 | 0.020 | 11.9 | 2.7–52.3 | 0.001 |
| Recent surgery | 9.5 | 2.0–45.9 | 0.005 | |||
| Hospitalization | 6.3 | 1.8–21.4 | 0.003 | 7.7 | 1.4–42.9 | 0.02 |
| Absolute contraindication to thrombolysis | 13.3 | 3.5–51.2 | <0.001 | 16.7 | 3.2–87.0 | 0.001 |
CI: confidence interval; OR: odds ratio.
Incidence of absolute contraindications for thrombolysis (n=22).
| Contraindications | n (%) |
|---|---|
| Central nervous system disease | 14 (63.6%) |
| Recent neurosurgery | 5 (22.7%) |
| Ischemic stroke <6 months previously | 4 (18.2%) |
| Previous intracranial bleeding | 3 (13.6%) |
| Central nervous system malignancy | 1 (4.5%) |
| Recent head trauma | 1 (4.5%) |
| Recent major surgery | 7 (31.8%) |
| Gastrointestinal bleeding <1 month previously | 1 (4.5%) |
In-hospital mortality was 36.5%. Although not reaching statistical significance, the reperfusion group presented 18.1% lower in-hospital mortality (27.8% vs. 45.9%, p=0.108), while the non-reperfusion group presented an increased relative risk for in-hospital mortality of 1.65 (95% confidence interval [CI] 0.88–3.11). The impact of reperfusion on hospital mortality is presented in Figure 2.
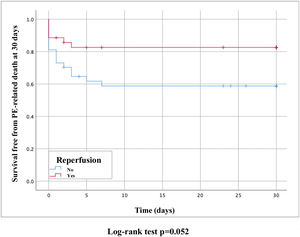
PE-related mortality at 30 days was 28.4% and reperfusion therapy presented a clinical benefit (17.1% vs. 40.5%, hazard ratio [HR] 0.39, 95% CI 0.2–1.0, p=0.052) (Figure 3).
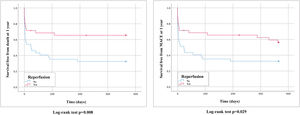
Follow-up at one year was available for 98.6% of the patients and one-year survival was 48.6%. Mean follow-up was 2.5±3.3 years, with overall survival of 33.8% (94.1% of patients had complete follow-up at 2.5 years). Of the 46 patients who survived to hospital discharge, there were seven cases of recurrent PE (15.2%) and three patients presented CTEPH (6.5%). The reperfusion benefit was more pronounced for long-term mortality, at 12 months (33.3% vs. 67.6%, HR 0.42, 95% CI 0.2–0.8, p=0.013) and during follow-up (51.4% vs. 81.1%, HR 0.42, 95% CI 0.2–0.8, p=0.005). Reperfusion also had a positive impact on one-year MACE (42.9% vs. 67.6%, HR 0.51, 95% CI 0.3–1.0, p=0.034) and MACE during follow-up (59.5% vs. 86.5%, HR 0.44, 95% CI 0.2–0.8, p=0.005). Long-term survival curves are displayed in Figures 4 and 5.
Most patients did not develop bleeding complications during hospital stay. There were 19 bleeding events (25.6%) (no information is available regarding 12 patients, either because they suffered very early death or because they were transferred to a different hospital). The 19 events were stratified in severity using the BARC classification: four were type 1 (no need for medical intervention), eight were type 2 (requiring medical evaluation/intervention), one was type 3a (decrease in hemoglobin of 3–5 g/dl), three were type 3b (need for surgical control of bleeding or vasoactive drugs), and three were type 5 (fatal bleeding): one central nervous system bleed and one hemoperitoneum in the non-reperfusion group and one central nervous system bleed in the reperfusion group. The reperfusion group showed a statistical tendency for higher-risk bleeding events compared to the non-reperfusion group: type 1 (6.1% vs. 6.9%); type 2 (24.2% vs. 0.0%); type 3a (3.0% vs. 0.0%); type 3b (6.1% vs. 3.4%); and type 5 (3.0% vs. 6.9%) (p=0.074). Overall, types 3c and 5 resulted in in-hospital mortality of 20% and 100%, respectively.
DiscussionThe main findings of this observational single-center cohort study of patients admitted with HR-PE over a decade (2008–2018) were: (1) HR-PE accounted for less than 5% of all PE events; (2) the reperfusion rate with systemic fibrinolysis as a first-line therapy was below 50%; (3) until 2018, the use of alternative reperfusion methods in HR-PE was minimal (less than 2%); (4) advanced age and the presence of absolute contraindications for thrombolysis were the main determinants for non-reperfusion; (5) the use of reperfusion therapy in HR-PE was associated with better short- and long-term survival.
In Portugal, data on the epidemiology of acute PE are scarce, especially in the high-risk group. The only available study reporting actual numbers in Portugal is by Gouveia et al.,2 who estimated a PE incidence in 2013 of 35/100000 population and overall in-hospital mortality of 17%. More recent data are not available and the frequency of HR-PE and the reperfusion rate in this group are not known. Hence, this is the first study reporting national numbers regarding these issues. In our study, the PE incidence was 58/100000 population, higher than previously reported, which is attributable to increased PE diagnosis due to improved diagnostic test sensitivity and the increasing longevity of the population. The incidence of HR-PE is in agreement with previously reported figures in studies in other countries.1–5
PE is the third leading cause of cardiovascular death worldwide, after acute myocardial infarction and stroke.1,13 RV failure secondary to a sudden increase in pulmonary vascular resistance is the main cause of PE-related mortality.14 The pathophysiological mechanisms behind this sudden increase in pulmonary vascular resistance are mechanical obstruction of the pulmonary arterial bed with one or several emboli and secondary vasoconstriction mediated by hypoxemia and production of local vasoconstriction mediators.15,16
Short-term PE-related mortality in HR-PE patients can exceed 30%.9 According to the 2019 ESC/ERS guidelines,1 thrombolysis is a class I recommendation, level of evidence B, for HR-PE. Its main clinical benefit is seen within 48 hours of symptom onset, but it can be used up to 14 days after the event.17 Although systemic fibrinolysis remains the first-line therapy in these patients,5 significant underuse is reported,6 as seen in our study. In the multicenter ICOPER registry,9 which used data on 2454 consecutive PE patients in 1995 and 1996 in 52 European and North American hospitals, two-thirds of patients were ineligible for fibrinolysis. More recent data from several other European registries report equally low reperfusion rates in these patients: in the Registro Informatizado de la Enfermedad Trombo Embólica (RIETE) study,18 only 20% of hemodynamically unstable patients were given reperfusion therapy. Likewise, in a German registry of 885806 PE patients, systemic thrombolysis was used in only 23% of hemodynamically unstable patients.8 In the latter registry, the HR-PE rate was 8.9% (slightly higher than in our study). Similarly to what is seen in other observational cohort studies, in which the HR-PE rate ranges between 3.5% and 6.1%,19,20 hemodynamically unstable patients may be under-represented due to under-diagnosis (more severe PE presentation may present with cardiac arrest and is an important cause of sudden death before a definitive diagnosis is possible). The reasons for non-reperfusion in the German study were also similar to those found in the present study: advanced age, recent surgery and cancer.8 In our study, age over 75 years presented 12 times the risk of non-reperfusion, and absolute contraindications to thrombolysis were also independent predictors of non-reperfusion.
Overall mortality over a mean follow-up of 2.5±3.3 years was very high (66.2%) in this HR-PE cohort. Our findings are in agreement with those observed in other studies, in which long-term mortality is also high, especially in the most severe forms of PE. Long-term mortality was 71.4% in Gupta et al.’s study,4 in which cancer-related death was the major contributor. Another common reason for long-term mortality in these patients is cardiovascular disease, which highlights the urgent need to implement long-term surveillance and prevention strategies for cardiovascular and thromboembolic events in the population with HR-PE.4,21
Despite the poor prognosis of these patients, our study supports the benefit of reperfusion as the standard of care in HR-PE, as it is associated with decreased 30-day and long-term mortality. This evidence is in line with what was previously shown in a meta-analysis of 15 controlled studies with 2057 PE patients randomized to systemic fibrinolysis and anticoagulation versus isolated anticoagulation. Reperfusion with systemic thrombolysis significantly decreased overall mortality compared to anticoagulation alone in hemodynamically unstable patients.22
Therefore, looking to improve survival in severe forms of PE, these data suggest that other reperfusion strategies need to be implemented besides systemic fibrinolysis. Considering the lack of access to emergent surgical embolectomy in this country, endovascular techniques have been increasingly seen as promising alternatives in higher-risk forms of PE.23,24 The 2019 ESC/ERS guidelines recommend CDT as class IIA, level of evidence C in HR-PE for patients in whom systemic thrombolysis has failed or is contraindicated, taking into consideration local experience and available resources.1 Most evidence regarding CDT comes from small prospective randomized controlled trials (ULTIMA)25 and single-arm studies (SEATTLE II,26 OPTALYSE PE,27 FLARE28 and EXTRACT PE29) that addressed intermediate-risk PE, a different clinical context from our study. However, all of these studies demonstrated the safety of CDT procedures and rapid improvement in RV function. In the prospective multicenter PERFECT registry,30 the clinical efficacy of CDT in 28 HR-PE patients was 86%, similar to that seen in the first cohort of mechanical thrombectomy performed in our center, between 2018 and 2020.31
All the above CDT studies showed a low incidence of major bleeding events and no fatal intracranial hemorrhage,25–29 suggesting that CDT could be extended to HR-PE patients with a high bleeding risk (such as the elderly and patients with active cancer, recent major surgery or intracranial diseases). Nevertheless, more data are needed to validate this strategy. Systemic thrombolysis is associated with major bleeding rates ranging between 9% and 24%, including intracranial bleeding,3,22 and these figures are also in line with those seen in our study. In a prospective registry, De Gregorio et al.32 used a combination of aspiration thrombectomy and low-dose catheter-directed thrombolysis as a first-line therapy in unstable PE; this was not only effective but also safe, with a very low rate of major bleeding (2.1%).
Wider use of CDT will require multidisciplinary expert PE response teams (PERTs) and the implementation of institutional and regional protocols that will enable appropriate risk stratification, timely signaling of higher-risk patients, and rapid delivery of reperfusion therapy when indicated.1,33 Access to PERTs leads to better clinical outcomes at 30 days, particularly reductions in mortality in severe forms of PE.34 It remains to be determined whether the use of reperfusion (pharmacological or endovascular) in a greater percentage of HR-PE patients can impact recurrent PE and/or evolution to CTEPH.
Study strengths and limitationsThere are several limitations to the present study that need to be mentioned.
Firstly, this is a single-center study, so its conclusions cannot be extrapolated to the national level.
Secondly, it is a retrospective observational cohort study, which leads to several limitations including the lack of treatment randomization, and the positive impact of reperfusion may also have been influenced by differences in baseline characteristics of patients in the two groups; the small sample size may also have limited the accuracy of the results. Furthermore, although the variables used to estimate disease severity (such as hemodynamic parameters and the PESI score) and predictors of reperfusion had high reporting rates, there are some missing variables in this study, notably data from laboratory tests that are not performed in the emergency department (such as N-terminal pro-brain natriuretic peptide). Reporting bias may have led to lower estimates of event rates (recurrent asymptomatic thromboembolic events or bleeding complications). Two foreign patients were lost to follow-up at 12 months and nearly 6% of patients had no clinical records after 2.5 years.
Finally, patients with sudden death due to PE before a definitive diagnosis could not be included in this registry, which may have led to overestimation of the reperfusion rate and underestimation of mortality.
Despite these limitations, this is the first study to report the reperfusion rate and its prognostic impact in HR-PE patients in a Portuguese tertiary center. Data were collected over eleven recent years and almost 100% of 12-month follow-up and 94% at the mean follow-up at 2.5 years were achieved, which are also considered strengths of the study.
ConclusionsIn line with previous population studies, the recommended reperfusion therapy was performed in only 50% of patients, with advanced age and absolute contraindications to fibrinolysis being the main predictors of non-reperfusion. Additionally, underuse of thrombolysis was associated with significantly higher short- and long-term mortality and cardiovascular event rates.
Conflicts of interestThe authors have no conflicts of interest to declare.