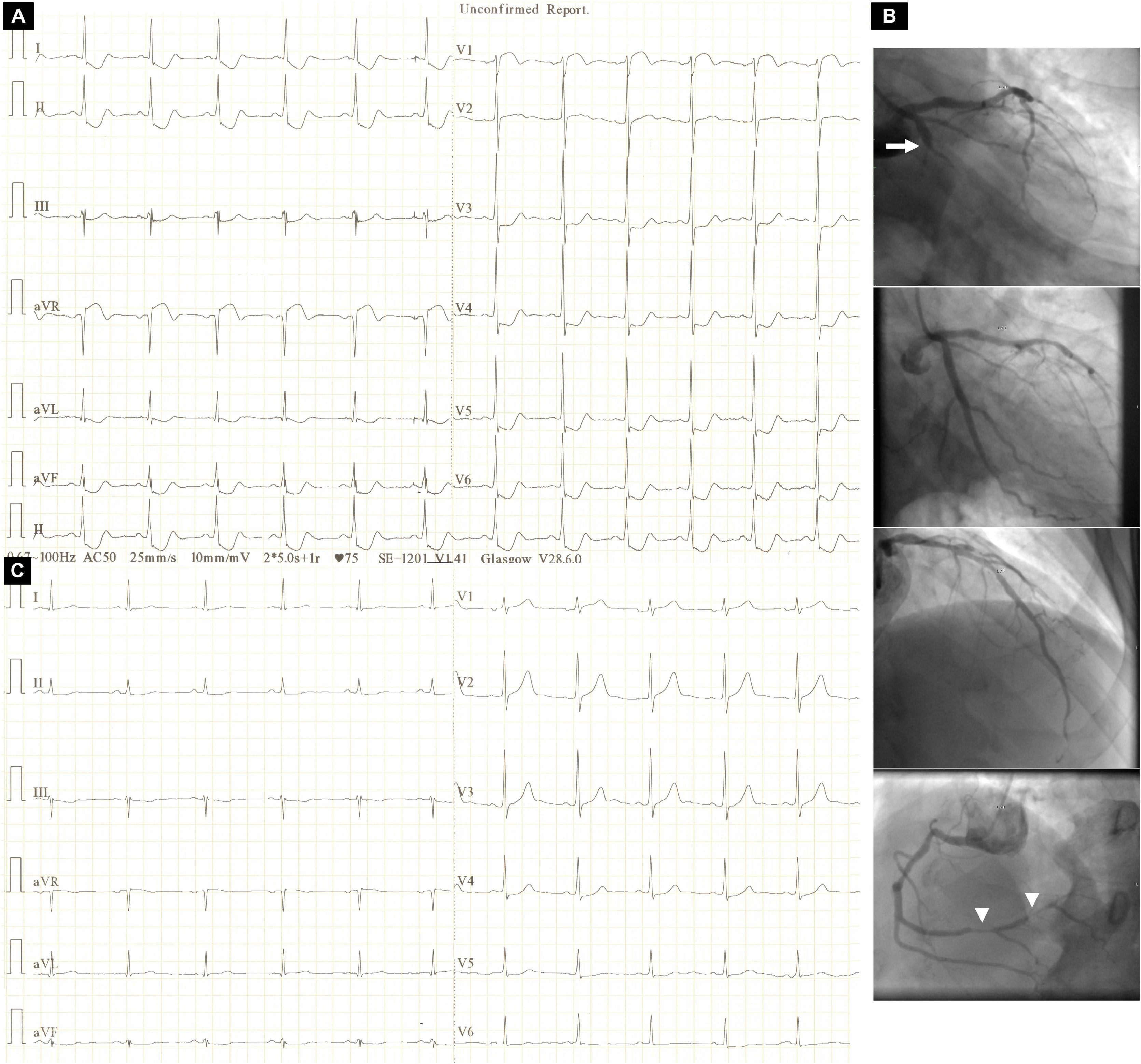
A 59-year-old man with a history of stent angioplasty of the left circumflex (LCx) artery presented with sudden-onset retrosternal chest pain associated with ischemic electrocardiographic (ECG) changes (Figure 1A). Emergency coronary angiography performed because of ongoing angina despite maximally tolerated therapy disclosed acute in-stent occlusion of the proximal LCx artery, successfully tackled with stenting (Figure 1B) resulting in resolution of the ischemic ST-segment changes (Figure 1C).
Electrocardiographic changes before and after stent angioplasty of the culprit lesion: (A) admission electrocardiogram depicting ST-segment depression at the J point in I, II, aVF, III and V3-V6 and ST-segment elevation at the J point in aVR and V1 (aVR>V1); (B) conventional coronary artery angiographic images depicting (top to bottom) acute proximal occlusion of the left circumflex artery (arrow), a good result after culprit lesion stenting, an unobstructed left anterior descending artery and high-grade lesions in the right coronary artery (arrowheads); (C) electrocardiogram after stent angioplasty of the culprit lesion depicting complete resolution of ST-segment changes and signs of inferior (QRS complex fragmentation in aVF and III) and lateral (R-wave amplitude and R/S amplitude ratio in V1 >3 mm and >0.5, respectively, and loss of R-wave height in V6) infarction.
In patients presenting with acute coronary syndrome, the ECG pattern comprising ST-segment depression in six or more leads, often with inverted T waves, and ST-segment elevation (≥0.1 mV) in aVR and V1 (aVR>V1) has been associated with circumferential subendocardial ischemia owing to subocclusive left main (LM) or three-vessel coronary artery disease (CAD).1 We present this ECG pattern in association with acute LCx artery occlusion, in which the superiorly directed ST-segment vector toward aVR is ascribed to ischemia, most pronounced in the basal inferior (formerly posterior) wall.2 Indeed, the post-angioplasty ECG showed fragmented QRS complexes in aVF and III as a manifestation of inferior infarction most pronounced in the basal inferior wall, which, owing to the fact that this area is the last to be depolarized, lacks a necrosis vector.3 Furthermore, the infarction extended to the basal lateral wall, as evidenced by a gain in R-wave height in V1 together with a loss of R-wave height in V6.4 In retrospect, absence of heart failure on admission and the presence of a final positive T wave in leads with ST-segment depression may be clues ruling out LM or three-vessel CAD.1,5
Conflicts of interestThe authors have no conflicts of interest to declare.