Coronary artery stent thrombosis is an uncommon but potentially catastrophic complication. The risk of very late stent thrombosis (VLST) raises important safety issues regarding the first generation of drug-eluting stents (DES). Although several complex mechanisms for VLST have been suggested and various predictors have been described, its pathophysiology is not completely understood and it is not known whether longer-term dual antiplatelet therapy reduces the risk. We present a rare case of simultaneous very late DES thrombosis in the three vascular territories, following discontinuation of antiplatelet therapy seven years after stent placement, presenting as cardiogenic shock.
A trombose de stent coronário é uma complicação rara mas potencialmente catastrófica. O risco de trombose muito tardia de stent (TMTS) tem levantado questões importantes sobre a segurança do uso de stents revestidos com fármacos (SRF) de primeira geração. Embora sejam vários e complexos os mecanismos da TMTS e vários os preditores que têm sido descritos, a sua fisiopatologia ainda não está totalmente esclarecida e desconhece-se se o prolongar da terapêutica antiagregante dupla reduz esse risco. Descrevemos um caso raro de trombose muito tardia de SRF ocorrendo em simultâneo nos três territórios vasculares, após interrupção da terapêutica antiagregante, sete anos após implantação dos stents, apresentando-se como choque cardiogénico.
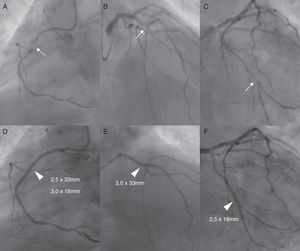
We present the case of a 49-year-old man with a past history of non-Q wave myocardial infarction (MI) seven years before, with three-vessel disease (right dominant circulation), who underwent complete functional percutaneous revascularization with sirolimus-eluting stents (Figure 1). He had an unremarkable follow-up under medical therapy, having completed 12 months of dual antiplatelet therapy (DAPT), with no residual angina, normal left ventricular ejection fraction and good functional capacity, although with suboptimal control of cardiovascular risk factors (dyslipidemia and overweight). No in-stent restenosis was noted in elective angiography at six months and he had no residual ischemia on exercise radionuclide myocardial perfusion imaging performed six years later.
Top: Left anterior oblique view of the mid-right coronary artery critical stenosis (Panel A), right anterior oblique cranial view of the mid-left anterior descending artery critical stenosis (Panel B) and right anterior oblique caudal view of the second obtuse marginal critical stenosis (Panel C). Bottom: Angiographic result after PCI with balloon predilatation and implantation of sirolimus-eluting stents (arrowheads) (D–F).
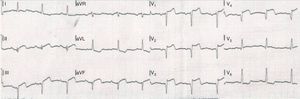
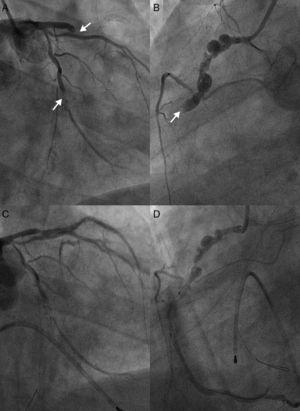
Seven years later, the patient was admitted with acute ST-segment elevation myocardial infarction, eight days after stopping aspirin. Also notable was a gout attack in the week of the cardiac event. He presented with typical severe retrosternal chest pain and ST-segment elevation in anteroseptal and inferior leads (Figure 2). He was immediately transferred to the cardiac catheterization laboratory, with a door-to-balloon time of 40 minutes and Killip class I on admission. Simultaneous stent thrombosis (ST) in the three coronary arteries was noted on first contrast injection (Figure 3). Thrombus aspiration and balloon angioplasty of the left anterior descending coronary artery achieved only suboptimal reperfusion. No-reflow and bail-out ensued, despite antithrombotic pharmacotherapy (aspirin and clopidogrel loading doses, unfractionated heparin and platelet glycoprotein IIb/IIIa receptor inhibitors) and intracoronary adenosine administration. Right ventricular pacing was needed due to complete atrioventricular block. Advanced cardiovascular life support was then started due to hemodynamic collapse and cardiac asystole. While cardiopulmonary resuscitation (CPR) was being performed, right coronary artery catheterization was attempted through a contralateral femoral approach with thrombus aspiration, balloon angioplasty and stent implantation. Despite intra-aortic balloon pump placement and prolonged CPR, the patient developed several episodes of ventricular fibrillation and incessant pulseless ventricular tachycardia and eventually expired.
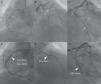
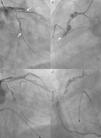
Dramatic simultaneous very late stent thrombosis of the left anterior descending, second obtuse marginal and right coronary arteries (A and B). Note the extensive positive remodeling with several aneurysms in the non-stented proximal right coronary artery reference segments (B and D). Suboptimal reperfusion and no-reflow phenomenon after thrombus aspiration, multiple balloon dilations, administration of intracoronary platelet glycoprotein IIb/IIIa receptor inhibitors and adenosine, right ventricular endocavitary pacing and intra-aortic balloon pump counterpulsation (C and D).
Coronary artery stent thrombosis is a rare but potential catastrophic complication of percutaneous coronary intervention (PCI), usually presenting as sudden death or MI.1,2 Moreover, patients who survive the event have a worse prognosis and are at increased risk of another ST in follow-up.3
The risk of very late stent thrombosis (VLST) (occurring more than one year after stent placement) raises long-term safety concerns regarding the first generation of DES (sirolimus-eluting and paclitaxel-eluting stents), even though, compared with bare metal stents, they were a major breakthrough, consistently being associated with reduced rates of angiographic restenosis and ischemia-driven target vessel revascularization.4 The reported cumulative rates of definitive VLST, according to the Academic Research Consortium definition, vary in the literature depending on the duration of follow-up used, and are slightly higher in observational studies.3–8
Delayed neointimal coverage and a hypersensitivity reaction to components of the drug-polymer-stent combination, together with ongoing vessel inflammation, impaired healing and abnormal remodeling leading to late and very late acquired stent malapposition, have been associated with VLST, but the precise mechanism is still unknown.9–13 Patient-related factors (including compliance with antiplatelet therapy), procedural and post-procedural factors (including type and duration of antiplatelet therapy) probably interact and predispose the patient to ST.2,3,10
Endothelial dysfunction has also been described as an adverse consequence following coronary DES implantation, although its clinical significance is still unknown.14 In fact, although DES target vascular smooth muscle cell proliferation and migration (neointimal hyperplasia), the key factors in the development of restenosis, they impair re-endothelialization and promote late in-stent neoatherosclerosis, a new concept whose precise mechanism is also unknown, that acts as another substrate for VLST.15,16 This also involves both proximal and distal non-stented reference segments and leads to delayed arterial healing, impairment of endothelial nitric oxide synthesis and imbalance between endothelium-derived relaxing and contracting factors, resulting in a thrombogenic environment and paradoxical vasoconstriction of the adjacent segments.10,16 Moreover, stent-induced mechanical changes in vessel geometry modify its response to endothelial injury and have been linked to the pathobiology of ST.17
To the best of our knowledge, this is the first report of a VLST, 81 months post-PCI, simultaneously occurring in the three coronary artery territories following discontinuation of antiplatelet therapy.
The peri-stent aneurysms depicted on angiography suggest extensive local positive vascular remodeling, a known late complication after DES deployment which has also been linked to very late acquired stent malapposition and ST; a review of the initial PCI showed no procedure-related mechanical problems such as residual dissection that could have acted as a substrate for the final extensive remodeling.18–20 Furthermore, although intravascular ultrasound was not performed due to the dramatic course of the event, the pronounced positive remodeling suggests the possibility of very late stent malapposition, a nidus for thrombus formation. Unfortunately there was no histopathological analysis of thrombus aspirates to search for eosinophilic infiltrates, which are common in thrombi from very late DES thrombosis associated with hypersensitivity reactions to the drug–polymer–stent combination, and related to the extent of stent malapposition.10
Several mechanisms have been implicated in this positive vascular remodeling, such as procedure-related acute vessel injury, hypersensitivity reactions to the stent polymer coatings, toxic effects of antiproliferative drugs on the vessel, and even focal infections.14–21
We hypothesize that a complex interplay of several thrombogenic factors probably acted together to create this “perfect storm” scenario that ended in a dramatic triple stent thrombosis: significant underlying endothelial dysfunction provoking an abnormal response to endothelial shear stress, abnormal rheological conditions and possible very late acquired stent malapposition; recent discontinuation of antiplatelet therapy (probably playing a pivotal role); and an inflammatory state – a recent gout attack, which has also been linked to acute MI.22
Our report highlights the serious consequences of discontinuing antiplatelet therapy, even several years after DES deployment and for a brief period. Although the optimal duration of DAPT after DES implantation is still unknown, robust data are only available for up to six months of therapy, and there are other mechanisms not related to discontinuation of drug therapy implicated in VLST, anecdotal cases like this demonstrate that there may be no time limit to the occurrence of this complication, and lifelong antiplatelet therapy is mandatory.2,9–12,23,24
Because there is currently no established method to identify patients at high risk of VLST, a recognized multifactorial event, and there is no definitive evidence on the optimal duration of DAPT, careful surveillance for potential late complications in patients with DES is crucial. Medication compliance, optimization of the PCI technique, quantification of coronary artery disease complexity by the SYNTAX score, shifting toward functional rather than visual guided angioplasty and thus avoiding unnecessary procedures, using invasive (fractional flow reserve, intravascular ultrasound, optical coherence tomography, and quantitative coronary angiography after intracoronary acetylcholine infusion for evaluation of vasomotor dysfunction) and non-invasive advanced coronary imaging techniques for tailored post-procedure monitoring, may help to reduce the incidence of this potentially devastating complication.25–27
The multifactorial nature of ST makes it difficult to predict and neither genotyping for reduced-function cytochrome P2C19 nor platelet function testing currently have a definite role.28
Although new anticoagulant and antiplatelet therapies have the potential to influence future clinical decisions regarding the duration of DAPT, the current approved therapies should not be discontinued ahead of the recommendations in the guidelines. The era of personalized antiplatelet therapy is therefore eagerly awaited.
Furthermore, although there appears to be a class effect between the different antiproliferative drugs, the data on new-generation DES (with thinner, more biocompatible and even bioabsorbable polymers, different stent alloys with better flexibility, conformability, and deliverability, and alternative drugs) are encouraging and may reduce this complication further.29,30
Finally, long-term follow-up studies are required to assess the significance and management of late and very late vascular pathologic changes secondary to DES implantation, particularly in-stent neoatherosclerosis, abnormal vasomotion, acquired very late stent malapposition and coronary artery aneurysms, and to improve our understanding of endothelial function.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.