A 57-year-old male with previously known severe primary mitral regurgitation was admitted to the intensive care unit (ICU) due to massive venous thromboembolism, associated with right ventricular dysfunction and two large mobile right atrial thrombi. Due to deterioration in his clinical condition despite standard treatment with unfractionated heparin, it was decided to use an ultra-slow low-dose thrombolysis protocol, which consisted of a 24-hour infusion of 24 mg of alteplase at a rate of 1 mg per hour, without initial bolus. The treatment was continued for 48 consecutive hours, with clinical improvement and resolution of the intracardiac thrombi and no complications. One month after ICU admission, successful mitral valve repair surgery was conducted.
This case demonstrates that ultra-slow low-dose thrombolysis is a valid bailout treatment option in patients with large intracardiac thrombi refractory to the standard approach.
Homem de 57 anos de idade, com insuficiência mitral grave primária previamente conhecida, é admitido na Unidade de Cuidados Intensivos (UCI) por tromboembolismo venoso massivo associado com disfunção do ventrículo direito e dois trombos auriculares direitos de grandes dimensões. Devido à deterioração clínica, apesar de instituído o tratamento clássico com heparina não fracionada, foi decidido administrar um protocolo de trombólise ultralenta em baixa dose, que consiste na infusão de 24 mg de alteplase durante 24 horas à velocidade de perfusão de um mg por hora, sem bólus inicial. O tratamento foi continuado durante 48 horas consecutivas, com melhoria clínica e resolução dos trombos intracardíacos, sem complicações. Um mês após a admissão na UCI, o paciente foi submetido a cirurgia de reparação valvular mitral com sucesso.
Este caso vem demonstrar que a trombólise ultralenta em baixa dose é uma opção de recurso válida em pacientes com trombos intracardíacos refratários ao tratamento clássico.
A 57-year-old male with dilated cardiomyopathy caused by severe primary mitral insufficiency presented to the emergency department with bilateral calf pain and increasing asthenia, dyspnea and orthopnea over the preceding week.
The patient was awaiting surgical mitral valve repair. Recently performed transthoracic echocardiography (TTE) had shown a myxomatous mitral valve with severe mitral insufficiency due to posterior leaflet prolapse with ruptured chordae tendineae, moderate tricuspid insufficiency, pulmonary hypertension with estimated pulmonary artery systolic pressure (PASP) of 68 mmHg, and good biventricular systolic function. He was chronically medicated with bisoprolol 2.5 mg daily, spironolactone 25 mg daily and furosemide 40 mg daily.
On examination, his blood pressure was 94/58 mmHg, pulse 92 beats per minute, respiratory rate 20 breaths per minute, oxygen saturation 97% in ambient air, and temperature 36.9°C. A holosystolic apical murmur was audible and bilateral crackles were present in the lower half of both pulmonary fields.
Laboratory workup showed normal blood gas analysis and normal serum lactate, hemoglobin 14.6 mg/dl, normal white cell count, elevated C-reactive protein (154 mg/l), acute kidney injury (serum creatinine concentration 1.92 mg/dl, previously normal) and elevated serum aminotransferases. Cardiac biomarkers exhibited elevation of brain natriuretic peptide (2737 pg/ml, previously 437 pg/ml) with no elevation of high-sensitivity troponin I.
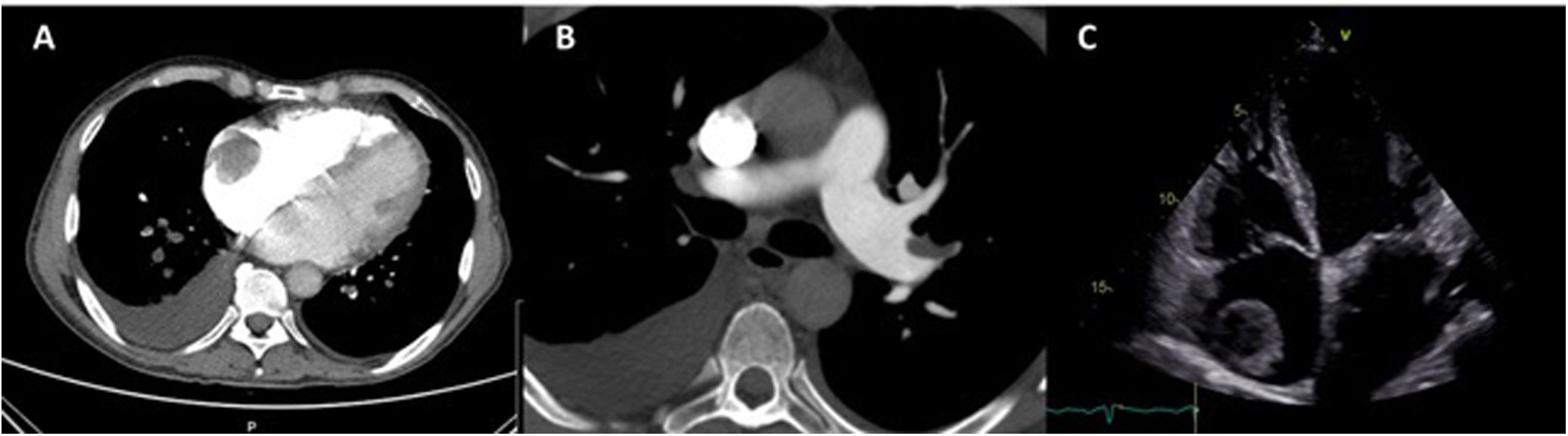
The electrocardiogram showed sinus rhythm with left atrial abnormality and positive electrocardiographic criteria for left ventricular hypertrophy. Computed tomography pulmonary angiography revealed pulmonary embolism (PE), with thrombi in the left segmental and subsegmental branches of the pulmonary artery and a 5-cm right atrial mass suggestive of thrombus (Figure 1A and B).
TTE showed severe mitral regurgitation caused by posterior leaflet prolapse, two mobile right atrial masses measuring 2.4 cm×1.5 cm and 3.6 cm×3.7 cm (Figure 1C), PASP of 70 mmHg, moderate dilatation of the right ventricle, reduced right ventricular function (tricuspid annular plane systolic excursion 16 mm) and preserved left ventricular function.
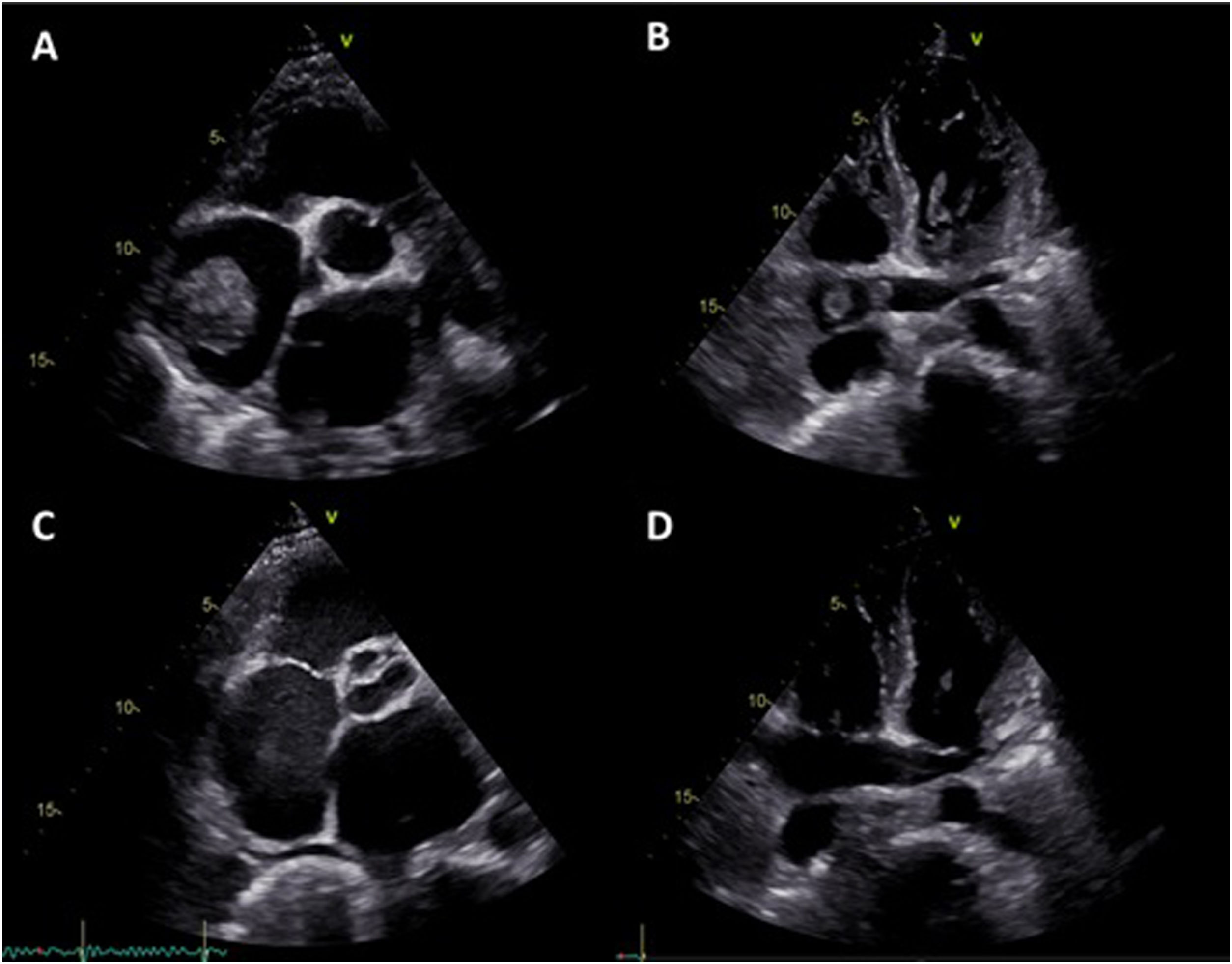
The patient was admitted to the intensive care unit (ICU) and started anticoagulation with unfractionated heparin (UFH). Despite five days with an activated partial thromboplastin time (aPTT) in the target range (60-80 s), the patient's clinical condition deteriorated and venous ultrasonography revealed extensive bilateral popliteal vein thrombosis. Repeat TTE showed severe deterioration of right ventricular systolic function and persistence of the right atrial masses with similar dimensions, together with mobile thrombi on the coronary sinus and the right pulmonary artery (Figure 2A and B). Urgent surgical thrombectomy was considered, but after consultation with the cardiac surgery team it was decided that the risk outweighed the benefit at that particular clinical juncture. After exhaustive discussion and literature review, it was decided to apply an ultra-slow low-dose thrombolysis protocol with a solution of 50 mg alteplase diluted in 100 mg isotonic saline at a rate of 1 mg per hour, without alteplase bolus, via a peripheral catheter placed in the right external jugular vein. The UFH infusion was maintained to a target aPTT of 50 s. After 24 hours of alteplase perfusion there was a moderate decrease in the atrial masses, as reported by TTE. The treatment was maintained for a further 24 hours and TTE was repeated, revealing a significant reduction in the right atrial masses with resolution of the coronary sinus and right pulmonary artery thrombi. Alteplase infusion was discontinued but the patient continued under treatment with UFH to a target aPTT of 60-80 s. Hemoglobin and platelet count remained stable during thrombolysis and there were no major bleeding complications. The patient was discharged from the ICU and started anticoagulation with warfarin, bridging with low molecular weight heparin. Seven days after alteplase discontinuation there was complete resolution of the intracardiac thrombi and 14 days later the patient was discharged from hospital under warfarin therapy (Figure 2C and D).
Transthoracic echocardiography four days after admission to the intensive care unit, showing persistence of the right atrial masses with similar dimensions (A) together with mobile thrombi on the coronary sinus (B) and the right pulmonary artery. Seven days after thrombolytic treatment there was complete resolution of the right atrial masses and thrombi on the coronary sinus and right pulmonary artery (C and D).
An investigation of prothrombotic states was performed, which excluded common genetic mutations prompting thrombosis (factor V Leiden, prothrombin, antithrombin, protein C or protein S gene mutations), antiphospholipid antibody syndrome and malignancy.
Approximately one month after ICU admission, a successful surgical mitral valve repair was conducted, with no postoperative complications. Three months after discharge, the patient is in New York Heart Association (NYHA) functional class I with no cardiovascular events or hospitalizations.
DiscussionRight atrial thrombi detected by echocardiography are found in up to 18% of patients with PE, and their presence confirms the diagnosis.1 The presence of intracardiac thrombi substantially increases the risk of right ventricular dysfunction and mortality, compared with PE alone.2 Various management strategies are used in clinical practice, including anticoagulation, thrombolysis, and interventional and surgical procedures.1,3–7 Unfortunately, the optimal management of right atrial thrombi remains controversial, since there have been no randomized controlled trials comparing the different treatment options.3
Systemic thrombolysis is recommended as the first-line treatment in patients with high-risk PE.1,5,7 In patients with intermediate-high-risk PE as defined by the European Society of Cardiology (ESC) guidelines, such as the patient in our report, anticoagulation therapy is deemed appropriate. In contrast to previous guidelines, according to which thrombolysis should be considered after weighing the potential bleeding risks and hemodynamic benefits, the recently published ESC guidelines do not recommend routine systemic thrombolysis, considering that it should be reserved for patients with hemodynamic deterioration.1,7–10 In this context, high-dose alteplase is currently the most widely used drug regimen, using a dose of 100 mg over two hours or 0.6 mg/kg over 15 min.1,7 Lower doses of alteplase have been studied in other conditions with good results and with lower bleeding risk. In another condition involving intracardiac thrombi, prosthetic valve thrombosis (PVT), slow low-dose alteplase infusion repeated as needed without a bolus was shown to be effective and safe compared to high-dose and rapid infusion regimens.11 The PROMETEE trial suggested that ultra-slow infusion of low-dose alteplase without bolus is associated with low non-fatal complications and mortality without loss of effectiveness in obstructive and non-obstructive PVT patients in NYHA functional class I-III.12 A successful case of ultra-slow low-dose thrombolysis applied in a patient with a mobile right atrial thrombus has been reported.13
In the present case report, a patient with known severe mitral regurgitation presented with massive venous thromboembolism with reduced right ventricular function and two large mobile right atrial thrombi with high risk of embolization (being attached to the right atrial wall) refractory to standard treatment with UFH.
Given the deterioration in his clinical condition and the prohibitive surgical risk, it was decided to use an ultra-slow low-dose thrombolysis protocol as a bridge to mitral valve repair surgery. The thrombolysis protocol used in this case was based on the PROMETEE trial protocol and consists of a 24-hour infusion of 24 mg of alteplase at a rate of 1 mg per hour, without bolus. The alteplase is started when aPTT is below 50 s. An echocardiogram is then performed to assess treatment success. Alteplase infusion is repeated if needed until a reduction of ≥75% in major diameter and/or thrombus area is achieved, up to eight times (maximum total dose of 200 mg). UFH infusion is maintained throughout the protocol, aiming for a target aPTT of 50 s.
This case demonstrates that ultra-slow low-dose thrombolysis is a safe and effective treatment option in patients with PE and large intracardiac thrombi.
Conflicts of interestThe authors have no conflicts of interest to declare.