Cardiovascular imaging plays an important role in the identification and characterization of the vulnerable plaque. A major goal is the ability to identify individuals at risk of plaque rupture and developing an acute coronary syndrome. Early recognition of rupture‐prone atherosclerotic plaques may lead to the development of pharmacologic and interventional strategies to reduce acute coronary events.
We review state‐of‐the‐art cardiovascular imaging for identification of the vulnerable plaque. There is ample evidence of a close relationship between plaque morphology and patient outcome, but molecular imaging can add significant information on tissue characterization, inflammation and subclinical thrombosis. Additionally, identifying arterial wall exposed to high shear stress may further identify rupture‐prone arterial segments. These new modalities may help reduce the individual, social and economic burden of cardiovascular disease.
A imagiologia cardiovascular tem desempenhado um papel importante na identificação e caracterização da placa vulnerável. Em particular, a possibilidade de identificar os indivíduos em risco de desenvolverem rutura de placa aterosclerótica e apresentarem síndrome coronária aguda é um objetivo importante. A deteção precoce de placas ateroscleróticas predispostas a sofrer rutura pode conduzir ao desenvolvimento de estratégias de intervenção farmacológica para reduzir eventos coronários agudos.
Procuramos rever o estado da arte da imagiologia cardiovascular na identificação da placa vulnerável. A evidência apoia a existência de uma forte relação entre a morfologia da placa aterosclerótica e a evolução clínica do doente, mas a imagiologia molecular pode adicionar caracterização tecidular e avaliação da inflamação e trombose. Também a identificação de locais na parede arterial expostos a elevada tensão de cisalhamento pode ajudar a identificar os segmentos arteriais mais predispostos a sofrer rutura. Estas novas modalidades de imagiologia podem contribuir para reduzir a carga individual, social e económica da doença cardiovascular.
Cardiovascular disease is the leading cause of death in the United States, Europe and most developed countries, with coronary artery disease being one of the main culprits. Atherosclerosis is recognized as the pathologic basis of this clinical entity but often remains clinically silent until narrowing of the arterial lumen becomes critical or an acute event is triggered by plaque erosion or rupture1; up to 60% of acute myocardial infarctions (MI) and sudden cardiac deaths occur as the first manifestation of disease.2 Accordingly, new screening methods are needed to identify patients at greater risk. The role of various imaging modalities in this setting has been tested over the years without definitive answers being reached. However, new techniques are emerging that may increase their diagnostic yield.
Predicting individual risk for developing acute coronary syndrome (ACS) in asymptomatic patients and, ultimately, predicting the risk associated with any single atherosclerotic plaque is a major goal in cardiovascular imaging. In particular, identification of atherosclerotic plaques prone to rupture may lead to the development of pharmacologic and interventional strategies to reduce acute coronary events.
In this review we focus on the current and future perspectives of cardiovascular imaging in the prevention and timely diagnosis of ACS, by identifying the vulnerable plaque and culprit lesions. These new modalities may help to further decrease ACS mortality and reduce the individual, social and economic burden of cardiovascular disease.
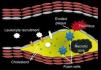
Pathophysiology of the vulnerable coronary plaque and targets for imagingThe components of atherosclerotic plaques include extracellular matrix (collagen and proteoglycans), cholesterol and phospholipids, inflammatory cells (macrophages and lymphocytes) and smooth muscle cells.3–6 Atherosclerosis primarily affects the intima but changes also occur in the media and adventitia.7 However, there is significant heterogeneity among atherosclerotic lesions depending on the relative quantities of the different plaque components.
Inflammation plays a major role in both initiation and progression of atherosclerotic plaque. Atherosclerosis appears to be a specialized inflammatory response in which leukocyte recruitment occurs in lesion‐prone areas of the arterial tree, subsequently leading to subendothelial accumulation of monocytes and lymphocytes. One of the earliest detectable cellular responses in atherosclerosis is adherence of leukocytes to the endothelium at particular anatomic sites of the artery wall.8–10 The recruited monocytes then differentiate into macrophages that accumulate lipids using scavenger receptors, ultimately becoming foam cells. These changes at the cellular level lead to the development of the fatty streak, an early precursor of atheroma.4
Progression of the atherosclerotic lesion occurs when foam cells and extracellular lipid droplets accumulate to form a core region, with a covering of smooth muscle cells and collagenous matrix, the fibrous cap. At this stage, immune cells including T cells, macrophages and mast cells continuously infiltrate the lesion.4,11,12 Many of these cells are activated and produce inflammatory cytokines that can promote plaque instability,13,14 a characteristic of the vulnerable plaque that can lead to thrombosis and to clinical events: plaque rupture is a major cause of thrombosis.15,16 Atherosclerotic plaques that are vulnerable to rupture have a dense inflammatory infiltrate17 and a thin fibrous cap.18 When plaque rupture occurs, the thrombus usually progresses to the deeper arterial layers and may lead to rapid growth of the plaque, disrupting normal blood flow.
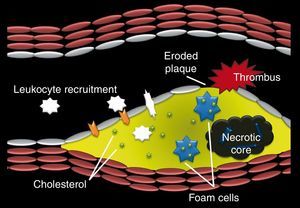
Another mechanism responsible for coronary thrombosis is plaque erosion.19 Eroded plaques are rich in smooth muscle cells and proteoglycans.19 When erosion occurs, thrombus adheres to the surface of the plaque, obstructing the lumen (Figure 1).
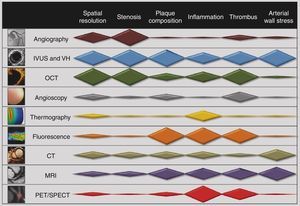
The morphologic characteristics of atherosclerotic plaques can be targeted by invasive and non‐invasive imaging modalities such as angiography, intravascular ultrasonography (IVUS), optical coherence tomography (OCT), computed tomography (CT) and magnetic resonance imaging (MRI). However, molecular imaging offers better discrimination of plaque components. To give an overall assessment of vulnerable plaque, imaging modalities need to target plaque morphology and structure, inflammation, apoptosis and thrombosis, and provide information on flow and wall stress (Table 1).
Cardiovascular imaging targets to assess vulnerable coronary plaque.
| Morphology and structure |
| •Stenosis |
| •Plaque cap thickness (thin vs. thick) |
| •Plaque cap disruption |
| •Remodeling |
| •Burden and pattern of plaque components (calcium, collagen, cholesterol, necrotic core, fibrofatty tissue and fibrotic tissue) |
| •Color (yellow plaque) |
| •Thrombus |
| Inflammation |
| Nonspecific markers |
| •Plaque temperature |
| •Plaque metabolic activity |
| Specific markers |
| •Adhesion molecules (VCAM‐1, ICAM‐1) |
| •Macrophage infiltration |
| •Protease activity in the plaque |
| Apoptosis |
| •Apoptosis in the plaque |
| Thrombosis |
| •Superficial thrombus (platelet aggregation and fibrin deposition) |
| Flow and wall stress |
| •Shear stress |
| •Arterial wall stress |
ICAM‐1: intercellular adhesion molecule 1; VCAM‐1: vascular cell adhesion molecule 1.
Angina is the clinical presentation of stable coronary artery disease and is usually present in patients with significant coronary artery obstruction at catheterization. However, these patients have been shown to have a fairly good prognosis with medical treatment.20 By contrast, ACS is associated with significant one‐year mortality.21
Although there is a subgroup of patients without occlusive atherosclerotic disease,22 MI is usually manifested angiographically by total or subtotal coronary occlusion of the infarct‐related artery.23,24 A pioneering retrospective study25 analyzing progression of coronary artery disease between two cardiac catheterization procedures showed that many patients with MI did not have significant coronary stenosis in the infarct‐related artery at baseline. It was therefore hypothesized that MI resulted from disruption of mild and moderate atherosclerotic plaques resulting in thrombus formation and vessel occlusion. Importantly, the study showed that coronary angiography may not be able to predict the location of a subsequent MI. Other anatomically‐based intravascular approaches have accordingly been developed in the quest to identify the vulnerable plaque. These include IVUS, OCT, angioscopy, near‐infrared spectroscopy (NIRS), intravascular photoacoustic (IVPA) imaging and intravascular MRI.
IVUS has been used to try to identify high‐risk plaques. Plaque features related to ACS include positive remodeling,26 rupture27 and hypoechoic plaque.26 Attenuated plaques, defined echographically as hypoechoic plaque with deep ultrasound attenuation in the absence of calcification, are also frequently found in patients with ACS.28 Histopathologic studies have shown microcalcification, cholesterol crystals and, importantly, thrombus.28,29 Moreover, the burden of attenuated plaque correlates with no‐reflow in ST‐elevation MI.30 However, the presence of nonculprit attenuated plaques that remain stable during follow‐up casts doubt on the usefulness of this parameter for predicting ACS.31
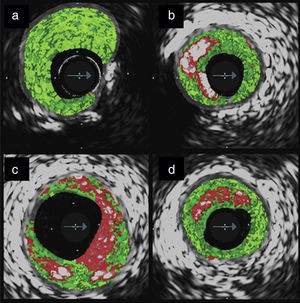
Although in‐vivo coronary artery histology is not feasible, radiofrequency IVUS analysis provides characterization of vessel wall components with high accuracy.32 The images obtained are usually color‐coded to discriminate dense calcium, the necrotic core, fibrofatty tissue and fibrotic tissue.33 According to the relative quantities of the components present, lesions may be classified as thin‐cap fibroatheroma, thick‐cap fibroatheroma, pathologic intimal thickening, fibrotic plaque or fibrocalcific plaque (Figure 2). In the PROSPECT trial,33 events unrelated to previous culprit lesions typically occurred at sites that were classified as thin‐cap fibroatheromas. Although promising and pioneering for in‐vivo detection of vulnerable plaque, these observations lack specificity; finding the three independent determinants of cardiac events simultaneously (thin‐cap fibroatheroma, minimal luminal area ≤4.0mm2 and plaque burden ≥70%), only predicted events in 18% of such plaques after three years. This imaging modality is thus not suitable for early clinical detection of vulnerable plaques. However, the PROSPECT trial is a good example of how these new imaging modalities should be tested.
OCT is an imaging modality that resembles IVUS but uses light instead of ultrasound to generate intravascular images. Its spatial resolution is about 10 times higher than IVUS but, due to its limited depth of penetration, imaging the vessel wall and plaque burden is often problematic. However, it can characterize plaque components, differentiating fibrous, fibrocalcific and calcific plaques.34 Additionally, OCT identifies disruption and erosion of the fibrous cap, intracoronary thrombus and thin‐capped fibroatheroma more accurately than IVUS in ACS patients, making it an attractive imaging method for the identification of vulnerable plaque.35 New and emerging OCT modalities such as polarization‐sensitive OCT, which provides assessment of plaque collagen content,36 may expand the capabilities of this intravascular imaging technique. However, large prospective studies are currently lacking.
Angioscopy is an intravascular imaging modality that allows direct visualization of the plaque surface and luminal thrombus. Patients with yellow plaques more frequently develop ACS.37 Yellow plaques are usually thin‐capped, exhibit positive remodeling, have a high lipid content and often present intraluminal thrombi.35,38 However, this technique has considerable limitations that limit its wider use, particularly the need for blood displacement and the subjectivity of plaque color assessment.
Other invasive imaging modalities currently under development that are promising for the morphologic detection of vulnerable plaques include NIRS, IVPA imaging and intravascular MRI. In the future, these techniques may improve intravascular tissue characterization, but their clinical relevance is still to be prospectively tested. NIRS is based on the absorption of light by organic molecules and is useful for identifying the chemical composition of tissues qualitatively and quantitatively.39 However, there is significant overlap between NIRS absorbance spectra, which reduces its specificity,39 and it is not a morphologic imaging modality. The latter limitation may be overcome by using a multimodality approach with IVUS.40 An IVUS catheter is also useful for photoacoustic imaging. With this modality, the tissue is irradiated by short laser pulses which generate tissue‐specific acoustic waves that can be detected by an ultrasound transducer. It may be particularly useful for spatially resolved assessment of the distribution of lipid deposits in the vessel wall.41 Intravascular MRI uses an intravascular magnetic resonance detector coil to enhance image quality, enabling characterization of the fine structure of deep small arteries. It reliably detected plaque composition and size in a validation study.42
Non‐invasive imaging modalities like CT and MRI are attractive tools for screening vulnerable plaques. Plaque extent and composition can be evaluated non‐invasively using coronary multislice CT. A study using coronary CT and IVUS found that calcified plaques were more prevalent in stable angina patients. By contrast, ACS patients had predominantly non‐calcified and mixed plaques.43 Evaluation of vulnerable plaque morphology by CT is hampered by lack of sufficient spatial resolution to identify thin‐capped fibroatheroma. However, thin‐capped fibroatheroma detected by IVUS frequently matches mixed plaques on CT.43 Early studies suggested that lipid and fibrotic plaque components can be differentiated by their attenuation coefficient,44 and these results were reproduced in post‐mortem histopathologic studies.45 However, the utility of this parameter is limited by the substantial overlap of attenuation ranges46 and by its low spatial resolution. Besides identifying low plaque density, CT can also identify positive remodeling and spotty calcification. These three CT characteristics of plaques are significantly more common in ACS than in stable disease.47 More importantly, a prospective study in 1059 patients followed for two years showed that low‐attenuation plaques with positive remodeling had a higher risk of developing into ACS.48 However, only 22% of patients with the two high‐risk CT features developed an acute coronary event. This shows that plaque characterization by CT is still not ready for clinical use in the identification of vulnerable plaque. However, scanner and contrast technology is evolving, and although a dramatic increase in spatial resolution cannot be anticipated in the near future, characterization of plaque components using targeted contrast agents may render this modality suitable for molecular imaging.
MRI images atherosclerotic plaque and the coronary lumen non‐invasively, without the use of ionizing radiation. It can rule out coronary artery disease with a negative predictive value of 88%.49 However, despite recent advances, MRI still provides a lower success rate and fewer interpretable segments than multislice coronary CT for the detection of coronary stenosis, with similar sensitivity but lower specificity.50 On the other hand, MRI can be safely performed serially, contrast agents may not be required and detection of luminal obstruction is not limited by heavy calcification.51 Additionally, black blood MRI imaging can provide fairly high spatial resolution images of the coronary artery wall. This modality is able to discriminate normal from diseased coronary artery segments and can quantify wall and plaque thickness.52 A substudy of the Multi‐Ethnic Study of Atherosclerosis (MESA) trial showed that MRI can prospectively detect positive coronary remodeling.53 However, MRI's full potential in the coronary arteries is limited by its modest temporal and spatial resolution. MRI of larger arterial territories has been shown to be highly accurate for measuring plaque size and to thoroughly characterize plaque composition,54 although this is not yet the case for coronary artery imaging. In the future, further improvements in external coils and the use of targeted contrast agents may provide better characterization of vulnerable coronary plaques.
In conclusion, there is ample evidence of a close relationship between plaque morphology and patient outcome. Although invasive modalities offer the highest spatial resolution, the best imaging technique is still to be determined. However, characterization of vulnerable plaque is not limited to morphologic assessment; molecular imaging can add significant relevant information concerning tissue inflammation and subclinical thrombosis (Figure 3).
Capabilities of cardiac imaging techniques for the assessment of different pathophysiological targets in vulnerable plaque. CT: computed tomography; IVUS: intravascular ultrasonography; MRI: magnetic resonance imaging; OCT: optical coherence tomography; PET: positron emission tomography; SPECT: single‐photon emission computed tomography; VH: virtual histology.
Traditionally, atherosclerosis was only diagnosed at advanced stages of the disease by documentation of stenosis or by perfusion assessment. However, new imaging modalities enable vessel wall composition to be assessed down to the cellular and molecular level. Plaque inflammation is strongly associated with plaque rupture and thrombosis,55 a hallmark of the ACS culprit lesion. Additionally, active and apoptotic leukocytes release enzymes which promote endothelial injury, cytolysis, and plaque disruption with subsequent thrombosis.55
Various invasive imaging modalities can be used to assess inflammation, thrombosis and apoptosis. They include thermography, OCT, angioscopy, IVPA imaging and IVUS.
Thermography is an invasive technique that evaluates temperatures at the plaque surface and is based on the principle that inflammation produces heat. Atherosclerotic plaque temperature and thermal heterogeneity correlate with plaque macrophage content,56 and in patients with acute coronary events thermography enables accurate localization of the culprit plaque.57 This is a promising approach to assess coronary inflammation indirectly. However, blood flow can attenuate thermal differences and should be systematically interrupted.
Macrophage infiltration within an atherosclerotic plaque can be accurately quantified using OCT. Ex‐vivo studies show a strong correlation between OCT signal variance and macrophage content of the fibrous cap.58 These results have been confirmed in human clinical studies.59 OCT thus has the potential to assess macrophage distribution in addition to obtaining high‐resolution cross‐sectional coronary images and may become a valuable imaging tool to determine plaque vulnerability.
Dye‐image angioscopy using Evans blue dye identifies damaged endothelial cells and fibrin,60 and may assist in the identification of intraluminal thrombi. Additionally, fluorescence imaging can be combined with angioscopy for molecular imaging of the vascular wall.61 Proteases can be targeted by optical prodrugs that are activated by enzymes. Cysteine protease activity has been imaged clinically62 and there are promising preclinical studies of assessment of plaque macrophage,63 VCAM‐164 and thrombus65 content.
Nanoparticles can be used to enhance the photoacoustic signal of macrophages in atherosclerotic plaques using IVPA imaging,66 which can also target matrix metalloproteinase activity.67
While IVUS is currently regarded as the standard modality for invasive structural imaging of the coronary arteries, it is still underdeveloped as a functional imaging tool. Contrast‐enhanced ultrasonography can be used as an intravascular molecular imaging modality by attaching contrast microbubbles to specific ligands. Intercellular adhesion molecule‐1, VCAM‐1, fibrin, fibrinogen and tissue factor are among the atheroma components that have been targeted in‐vivo using IVUS.68 Combining IVUS‐based molecular imaging with highly detailed anatomic information is a promising way to assess vulnerable plaque invasively.
Non‐invasive imaging modalities are probably the most studied techniques for molecular imaging of inflammation, apoptosis and thrombosis. Nuclear imaging provides tools that target specific or nonspecific inflammation in atherosclerotic plaques. Fluorodeoxyglucose positron emission tomography (PET) measures glycolysis to assess arterial metabolic activity.69 Fluorodeoxyglucose PET signal intensity correlates with the concentration of macrophages in human atherosclerotic plaques.70 In ACS patients, metabolic activity by PET is detected both within the culprit lesion and in the ascending aorta and left main coronary artery,71 suggesting that PET can be used clinically to target atherosclerotic plaque inflammation. Additionally, the PET contrast agent 18F‐4V has been shown to enable non‐invasive imaging of VCAM‐1 in inflammatory atherosclerosis.72 The specific accumulation of leukocytes in tissue can also be targeted. Nanoparticles are often used as CMR contrast agents to image macrophage infiltration in atherosclerotic lesions but they can also be used with nuclear tracers, with higher sensitivity.63 These modalities have the potential to elucidate the in‐vivo kinetics of inflammatory cell infiltration in atherosclerotic plaques. Furthermore, the combination of the high sensitivity of PET with the anatomic detail provided by CT or CMR73 in hybrid imaging may locate inflammation.74,75 These hybrid approaches allow more information to be derived from a single exam.
Nuclear imaging can also target atheroma proteases, which have been implicated in plaque destabilization. Radiolabeled small‐molecule protease inhibitors have been tested in mice models of atherosclerosis76 and have the potential for translation to clinical practice in the future.
Nuclear imaging may also be useful in detecting apoptosis. Preliminary data on technetium‐99m‐labeled annexin A5 in PET‐CT suggests it may be a new method for assessing plaque instability and identifying patients at risk for acute vascular events.77 However, prospective data in coronary artery disease are lacking.
Although the identification of subclinical thrombus adhering to vulnerable plaque would be of enormous value in the detection of plaques at the highest risk for progression to an acute coronary event, in reality this is not currently feasible. However, exploratory investigations in animal models suggest that nuclear imaging may be valuable in this regard; 99mTc‐labeled DMP‐444, a compound that binds to glycoprotein IIb/IIIa, identified microthromboemboli in a canine model of acute MI.78
MRI also has the ability to detect inflammation in atherosclerotic plaques. Detection of macrophage infiltration in plaque by MRI relies on the fact that nanoparticles are taken up by activated macrophages in vulnerable plaques. Such nanoparticles include liposome‐encapsulated gadolinium, which increases T1 signal intensity, and ultrasmall superparamagnetic iron oxide (USPIO) agents that enhance T2* contrast.79 USPIO agents already enable direct in‐vivo visualization of macrophage infiltration in carotid atheroma.80,81 Additionally, gadolinium‐based activatable sensors allow assessment of myeloperoxidase activity by MRI82 and VCAM‐1 contrast agents are under development.64
Apoptosis is also detectable by MRI, although it has mainly been evaluated in the myocardium. Late gadolinium enhancement MRI is used clinically to detect myocardial infarct size but it cannot differentiate necrosis from apoptosis. A combined MRI approach with an annexin V‐targeted nanoparticle and delayed gadolinium enhancement can simultaneously detect necrosis and apoptosis.83 This approach may be particularly useful because apoptosis is a reversible phenomenon that can be therapeutically targeted.
Plaque‐associated angiogenesis is another feature related to plaque initiation and rupture that can be assessed using MRI paramagnetic nanoparticle contrast agents targeting αvβ3‐integrins.84 Similarly, molecular MRI of intravascular clots is feasible; the fibrin‐targeted contrast agent EP‐2104R identified human clot material in a swine model of coronary thrombosis.85 This contrast agent is in an advanced stage of development and has successfully passed a human phase II trial.86
An iodinated nanoparticulate contrast agent has also been developed for the non‐invasive detection of macrophages using cardiac CT.87 This contrast agent may provide additional information but its discriminatory value in highly calcified lesions is not known.
In conclusion, there is broad evidence supporting a role for molecular imaging in detecting vulnerable plaque both invasively and non‐invasively. However, much of the data come from preclinical studies. These modalities now face the challenge of translation to a clinical setting.
Assessment of shear stress and coronary wall properties for predicting vulnerable plaque locationAtherosclerotic lesions show a focal distribution with preference for arterial branches and curvatures and there is thus an association with local hemodynamic factors. It is now well established that wall shear stress (WSS), defined as the mechanical force imposed on the endothelium by the flowing blood, plays a significant role in endothelial homeostasis and is implicated in the distribution of atherosclerotic lesions.88 Hemodynamic flow typical of atherosclerosis‐prone segments causes activation of the NF‐κB pathway and increases the expression of proinflammatory cytokines and adhesion molecules.89 Hemodynamic strain may thus initiate and propagate the arterial inflammatory process.
Computational fluid dynamics is regarded as the gold standard for determining WSS. It requires coronary imaging that may be obtained by various methods, including non‐invasive modalities such as ultrasound90 and MRI91 and invasive methods such as IVUS92 and intravascular Doppler ultrasound.93 Although low or oscillating WSS has been associated with plaque initiation,88 localized high WSS may be a trigger for fibrous cap rupture.94 Accordingly, any of the above imaging modalities may be useful for predicting localization of atherosclerotic plaques exposed to high WSS and therefore prone to rupture.
The clinical use of shear stress imaging is in its infancy. The recently published PREDICTION study,95 which used IVUS profiling to estimate shear stress in the coronary arteries, elegantly showed that low endothelial shear stress can independently predict plaque progression and luminal narrowing. However, the small number of clinical events precluded firm clinical conclusions from being drawn; our knowledge may be expanded by studies using larger sample sizes and longer follow‐up times.
Palpography measures the rate of plaque deformation in response to the pulsating force of blood flow. It is measured using a cross‐correlation analysis of radiofrequency ultrasound signals recorded at different intravascular pressures.96 The tissue strain may be color‐coded on the arterial wall97 and high strain patterns have been more frequently identified in ACS patients.98 Palpography is less technically challenging than shear stress measurement and also holds considerable promise for the identification of vulnerable segments in coronary arteries.
The integration of structural, molecular and shear stress imaging information may be useful for integrated risk assessment of plaque vulnerability. In this regard, IVUS imaging, by potentially providing integrative data, is one of the most attractive imaging options (Figure 3).
ConclusionAs discussed above, there are specific advantages and disadvantages associated with each modality for imaging vulnerable atherosclerotic plaque in the coronary arteries. Certain technical and patient security challenges need to be overcome by molecular imaging modalities before they can become clinical tools. These imaging techniques should be tested in clinical trials to assess their real‐world prediction of coronary events; they are promising but, ultimately, the ability to identify patients who will benefit from intensive medical therapy, local intravascular therapy or preventive coronary bypass surgery will determine their success.
Multimodal imaging integrating coronary morphology, wall composition, characterization and wall shear stress can provide a highly accurate and predictive assessment of vulnerable plaque. For determining this integrated individual risk, invasive imaging modalities currently present various advantages over PET, MRI and CT; they provide greater anatomical detail, have tissue characterization capabilities and, particularly IVUS, assess inflammation and thrombosis using molecular imaging in addition to localizing areas of high wall stress.
In conclusion, much of the impetus for the development of new imaging modalities has come from basic science. In order to predict individual cardiovascular risk and to develop pharmacologic and interventional strategies to reduce acute coronary events, integrative clinical translation is crucial to the future success of vulnerable plaque imaging.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.