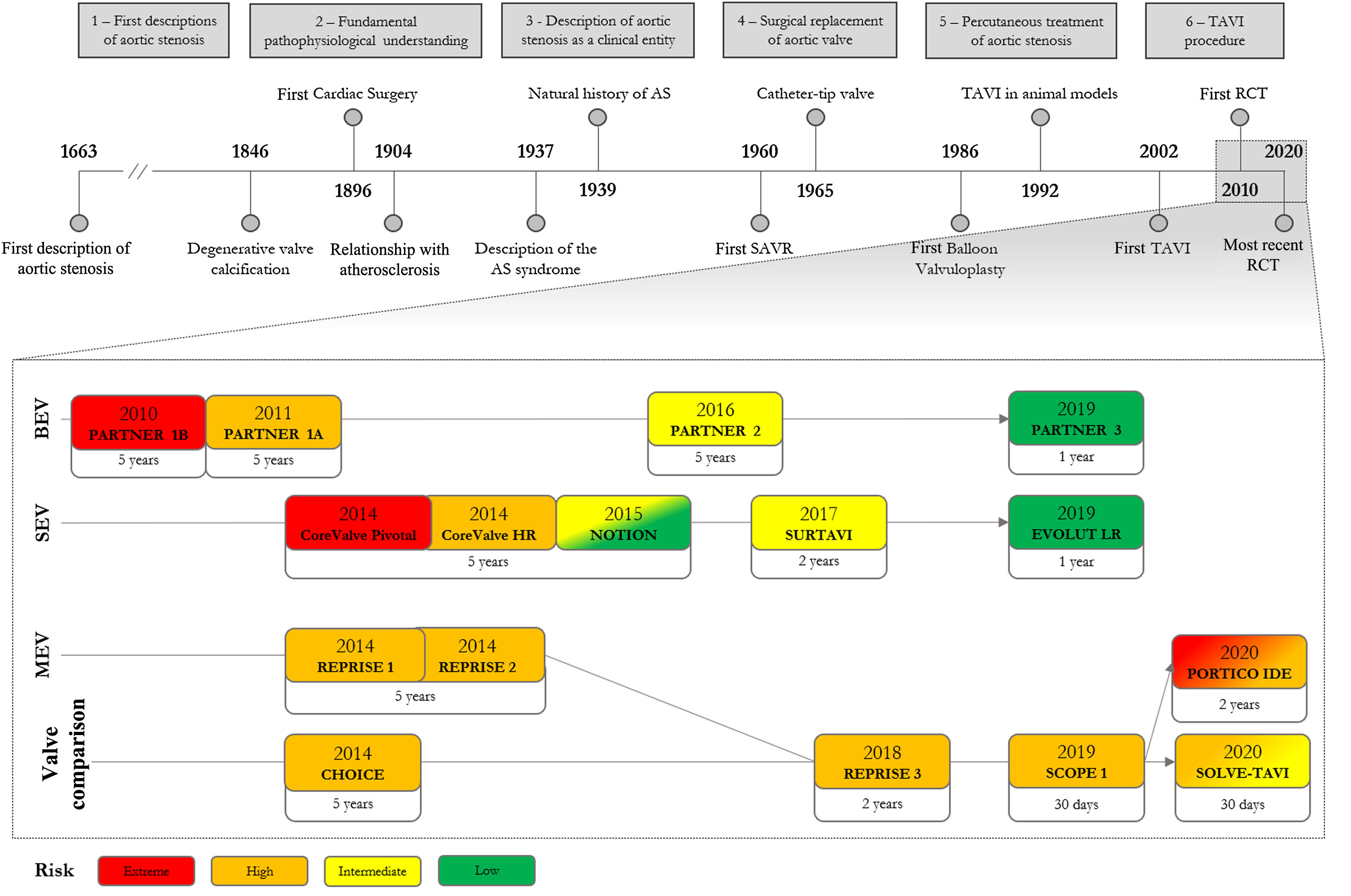
Nearly 300 years after the first description of aortic valve obstruction, it has taken less than two decades of randomized clinical trials (RCTs) for transcatheter aortic valve implantation (TAVI) to become a recognized strategy for patients with aortic stenosis.
The high density of recent publications makes it easy to ignore the history that led to the development of this procedure. Knowing the evolution of a diagnostic or therapeutic technique improves critical reasoning, prevents repeated mistakes, paves the way for future research and contributes to an insightful perspective on the subject. Nevertheless, it should not overshadow the findings of recently published RCTs, which still are the mainstay of clinical practice.
In this timeline review, the authors aim to recap the development of TAVI, combining the pathophysiology of aortic stenosis and the initial concept of TAVI with the roadmap of clinical trials that led to the generalization of the TAVI procedure.
Após cerca de 300 anos da primeira descrição de uma obstrução da válvula aórtica, menos de duas décadas de estudos randomizados decorreram até a válvula aórtica percutânea (VAP) ser uma estratégia terapêutica reconhecida para doentes com estenose aórtica.
Com a elevada densidade de estudos recentes é possível negligenciar a perspetiva histórica que originou este procedimento. Conhecer o fundamento histórico que sustenta uma técnica terapêutica ou diagnóstica melhora o raciocínio clínico, previne a repetição de erros do passado, define os estudos do futuro e permite enriquecer a perspetiva sobre o tema. No entanto, o conhecimento histórico não se deve sobrepor aos estudos randomizados mais recentes, que continuam a ser o pilar principal das decisões feitas na prática clínica.
Nesta revisão temporal, pretende-se delinear uma linha temporal da história da VAP, combinando o desenvolvimento do conhecimento da patofisiologia da estenose aórtica e a sequência de descobertas que originou o conceito inicial da VAP, com o percurso de estudos clínicos que levaram à globalização deste procedimento.
Given the negative impact of time constraints in clinical practice, and the exponential growth of publications on transcatheter aortic valve implantation (TAVI), it is tempting to focus attention on the most recent data, ignoring the history that led to the development of this procedure. However, understanding the major breakthroughs of the past not only improves critical thinking, but also avoids repeated mistakes and paves the way for future research.1
In this article we aim to identify the main timeline of our understanding of the pathophysiology and management of aortic stenosis (AS) that ultimately led to the TAVI procedure, and the cascade of clinical trials that assured its position in the treatment of AS.
Historical overview of aortic stenosisThe first TAVI procedure was performed in 2002 by Alain Cribier,10 but the history of TAVI starts with the history of AS. Arguably, the first description of aortic valve obstruction was given in 1663 by Lazare Riviére,3 in which he characterized a patient with progressive dyspnea, leg edema and a “pulse small, irregular, with every variety of irregularity” – probably a patient with heart failure, atrial fibrillation and the weak and late (parvus et tardus) peripheral pulse of AS. Despite medical therapy, the patient's condition progressively worsened until he “suffered from the greatest difficulty in breathing (…), the “swelling of the legs increased so that the thighs were involved” and on “the eighth day, he died in the hours of evening”. In the autopsy report, Riviére described that “in the left ventricle of the heart, round caruncles were found like the substance of the lungs, the larger of which resembled a cluster of hazel nuts and filled up the opening of the aorta”. These clusters were most likely vegetations on the aortic valve, projecting into the aorta.4
This connection between endocarditis and calcification of the aortic valve was unquestioned until the first half of the 19th century.5 In 1846, Charles Ewald Hasse stated that “ossification cannot invariably be ascribed to endocarditis” and proposed a degenerative process behind aortic valve calcification. Over the years, this degenerative etiology was increasingly recognized and, in 1904, Möenckeberg described the histological features of valve degeneration and explained the “close relationship between this disease and the senile form of atherosclerosis”.6
In the first half of the 20th century, AS was established as a distinct clinical entity. In 1937, Contratto and Levine recognized angina, dyspnea and syncope as the cardinal symptoms of the disease and sudden death as one of the three major causes of death (the others being endocarditis and heart failure).7 In 1939, Dry and Willius stated that “symptoms are likely to remain in abeyance for many years, but with the onset of myocardial failure the outlook becomes serious”, suggesting that a clinically silent period of the disease preceded the onset of rapidly progressive and difficult-to-treat heart failure.8 In 1964, Ross and Braunwald identified a link between the clinical syndrome and prognosis, with heart failure presaging the worst life expectancy.9 During that era, left ventricular dilatation and dysfunction was associated with rapid disease progression10 and patients with heart failure and preserved left ventricular function (diastolic dysfunction) were recognized as having a different survival outcome.11
Surgical aortic valve replacement (SAVR) evolved alongside characterization of the clinical syndrome. After the first heterotopic valve implantation in 1952 by Charles Hufnagel, the advent of heart-lung machines with extracorporeal circulation enabled the first annular SAVR to be performed in 1960 by Dwight Harken.12–14 Following poor long-term results with valvuloplasty (decalcification, commissurotomy and other procedures that attempt to restore the valve cusps) and prosthetic tricuspid implants, the first successful SAVR was performed with a ball-cage valve. Combined with other small series, Effler et al. published a study of 224 patients with satisfactory results with the Starr-Edwards ball-valve prosthesis, favoring SAVR as the first-line therapy for severe AS,15 although high operative mortality was the principal limiting factor to the procedure, mostly related to the complexity of the operation and perioperative care.16 It became clear that the benefits of SAVR needed to be balanced against the risks of the procedure, with intervention being reserved for cases in which there was likely to be prognostic benefit.
The focus therefore turned to risk stratification and patient selection. This considered, on one hand, the prognostic benefit of surgery: it was consensual that the prognosis after symptom onset was ominous,17,18 as opposed to the lack of agreement concerning asymptomatic patients.19–25 After 1968, SAVR was recommended after the onset of symptoms in patients with AS.26 On the other hand there was the patient's surgical risk: a significant number of higher-risk patients were not suitable for surgery. In the 1980s, age over 70 years was considered a high-risk feature for cardiac surgery,26 and subsequently the EuroHeart survey revealed that nearly one third of symptomatic patients were not suitable for valvular surgery.27 Therefore, a less invasive approach was required for this large group of inoperable patients.
The concept behind transcatheter aortic valve implantationThe concept of percutaneous valves is not new. The first percutaneous aortic valve was implanted almost half a century before the first TAVI procedure. In 1965, Davies and colleagues used a parachute-like temporary aortic valve, implanted under the aortic annulus, to stabilize a patient with severe aortic regurgitation, as a bridge to SAVR.28 Catheter-mounted valves were studied in animal models of acute aortic regurgitation,29,30 but the system did not evolve into a full-fledged implantable valve.
The first percutaneous treatment for AS took place in 1985, when Alain Cribier performed the first transcatheter aortic balloon valvuloplasty in a 72-year-old patient. The initial series showed promising results, with a significant increase in valve area, reduction in aortic gradients, rapid improvement of performance status and no severe complications.31 The short- to medium-term effects of valvuloplasty were interesting, but it became clear that it was still associated with significant short-term morbidity.32 The restenosis rate at one year was high,33–35 and the natural history and prognosis of AS patients did not change.36 Thus, a more definitive, long-term solution was required.
From the routine observation that balloon valvuloplasty could open calcified aortic valves in a circular fashion, allied with the stentless bioprosthesis technology from SAVR, the initial concept emerged of an expandable stent containing a valve that could be implanted inside the native aortic valve.26 The first successfully implanted transluminal stented valve in an animal model was developed by Henning Rud Andersen, who prepared a porcine aortic valve in an expandable metallic stent compressed around a deflated balloon catheter.37 Unpublished autopsy studies by Cribier showed that such stents could be easily opened and would adhere to the calcified aortic valve.2
In the 1990s and early 2000s, animal studies confirmed that implantation of such valves was a feasible and safe procedure, without the risk of coronary flow obstruction if correct valve orientation and implantation plane were assured.37,38 The first transluminal stented valve in humans, however, was implanted in 2000, when Philipp Bonhoeffer placed a stented valve in a pediatric patient with a degenerated right ventricle-to-pulmonary artery conduit.39
On April 16, 2002, Cribier and colleagues performed the first TAVI in a 57-year-old patient with severe AS and refractory cardiogenic shock.2 The patient had a left ventricular ejection fraction of 14%, complicated with subacute leg ischemia due to severe peripheral artery disease and an occluded aortofemoral bypass. He was not eligible for cardiac surgery and after failed sustained results with balloon valvuloplasty, was proposed for TAVI. The bovine valve (three pericardial leaflets), mounted within a balloon-expandable stent, was implanted under conscious sedation with an anterograde transseptal approach through the femoral vein. No major complications were reported following the procedure. Minimal paravalvular regurgitation (PVR) was observed with normal mitral valve function and coronary flow. At 48 hours, the valve displayed excellent function with gradual improvement of hemodynamic and echocardiographic features. Nonetheless, the patient died 17 weeks after the procedure due to non-cardiac complications.
Original studies on high-risk patients undergoing transcatheter aortic valve implantationThe first successful TAVI was the real-life proof of concept that set the tone for the main randomized controlled trials (RCTs), which initially focused on the feasibility of TAVI in extreme-risk patients. These trials can be classified according to the valve delivery system: balloon-expandable valves (BEV, mainly Edwards® SAPIEN), self-expanding valves (SEV, mainly Medtronic® CoreValve) and mechanically-expandable valves (MEV, mainly Boston Scientific® Lotus). Furthermore, patients can be divided according to risk strata into extreme/inoperable, high-, intermediate-, and low-risk, based on the EuroSCORE I or II, or Society of Thoracic Surgeons Predicted Risk of Mortality scores.
The first trials for inoperable patients were cohort B of the PARTNER trial (PARTNER B) (2010)40 to assess BEV and the CoreValve Extreme Risk Pivotal Trial (2014)41 for SEV. In PARTNER B, patients were randomly assigned to receive TAVI or optimal medical therapy, while in the non-randomized CoreValve Pivotal Trial, TAVI was compared with a prespecified objective performance goal. Both valve systems clearly improved symptoms, aortic area and gradients and, most significantly, decreased mortality at one-year follow-up.
Results from PARTNER B were sustained up to five years, with symptomatic relief and decreased mortality. Moreover, valvular structure and function were not significantly disrupted. However, in this landmark trial, TAVI was associated with a higher 30-day risk of stroke.42–44
Cohort A of the PARTNER trial (PARTNER A) (2011)45 and the U.S. CoreValve High Risk Study (2014),46 for BEV and SEV, respectively, targeted high-risk surgical patients. Both randomized up to 700 patients to TAVI or SAVR with a bioprosthetic valve. In both trials, TAVI was non-inferior to surgery regarding all-cause mortality at 30 days and at one-year follow-up. Major stroke rates were similar in both procedures. Each strategy was associated with a different subset of complications: TAVI was prone to vascular complications, significant PVR and pacemaker implantation, while SAVR was associated with major bleeding, acute kidney failure and atrial fibrillation.
Both trials have published data up to five-year follow-up, with similar functional and mortality endpoint results.47–51 These trials also revealed a significant association between moderate-to-severe PVR and increased long-term mortality.
Progression to lower-risk patientsThe PARTNER 2 (2016)52 and SURTAVI (2017)53 trials addressed the intermediate-risk group. PARTNER 2A used the SAPIEN XT valve (BEV), while SURTAVI used the CoreValve or Evolut-R valves (SEV). Both trials randomly assigned up to 2000 patients to TAVI or SAVR and followed them for 24 months. These valves were associated with similar short- and long-term mortality in SAVR and TAVI patients. The tendency towards different complications following TAVI and SAVR remained the same in these intermediate-risk patients and similarly, significant PVR was associated with long-term mortality. At five years of follow-up, death and stroke endpoints remained similar between the groups in the PARTNER 2 trial. Still, rehospitalization, reintervention and at least mild PVR was more frequent in the TAVI group.54
The first trial to include low-risk patients was NOTION (2015)55 for SEV. This study of 280 patients in northern Europe (82% low-risk patients) displayed comparable results regarding all-cause mortality and other major endpoints for TAVI and SAVR at one- and five-year follow-up. Although valve areas and gradients were better in TAVI at five-year follow-up, PVR and pacemaker implantation were significantly more frequent.56
PARTNER 3 (2019)57 and an interim analysis of the Evolut Low Risk Trial (2019),58 for BEV and SEV respectively, enrolled low-risk patients. Evolut Low Risk employed a 24-month primary endpoint (death or stroke) and the results were inferred using Bayesian statistics. Both trials displayed low mortality at one-year follow-up for both TAVI and SAVR. Interestingly, in PARTNER 3, there were no significant differences between the two procedures concerning the classic adverse effects (pacemaker implantation within 30 days, significant PVR and major vascular complications). Nonetheless, new conduction disorders, such as left bundle branch block, and mild PVR was still more frequent following BEV. Importantly, only one-year valve durability results are available in low-risk, usually younger, patients.
Development of new delivery methodsMEV were developed in order to decrease significant PVR and to increase the implantation accuracy associated with early TAVI models. These valves are repositionable and retrievable when in final position and contain an adaptive polyurethane/polycarbonate outer seal to minimize PVR. REPRISE (2014)59,60 and REPRISE II (2014)61–63 were single-arm studies that assessed MEV in a total of 131 patients. All the valves were correctly implanted and partial or total resheathing was successfully performed in 40 patients in total. No moderate-to-severe PVR was seen at one-year follow-up.
Studies directly comparing transcatheter aortic valve systemsCross-trial comparisons between valve systems are potentially hazardous, due to differences in population selection and trial designs.
The CHOICE trial (2014)64–66 randomly assigned 241 patients to receive the SAPIEN XT or CoreValve. The BEV displayed higher implantation success due to a lower rate of valve replacement and moderate-to-severe PVR compared to the SEV cohort, but forward flow valve dynamics were superior with the SEV. No significant differences regarding hard endpoints were seen in up to five years of follow-up.
REPRISE III (2018)67,68 randomized up to 900 patients to receive a MEV or SEV (CoreValve Classic in about half of the patients). This non-inferiority trial showed similar mortality at two-year follow-up. The MEV showed better results in terms of disabling stroke, valve migration, need for reintervention, functional class and PVR. Conversely, the SEV revealed better valvular areas and aortic gradients, in addition to lower rates of thrombosis and pacemaker implantation.
The SCOPE I trial (2019)69 randomly assigned up to 700 high-risk surgical patients to a BEV (SAPIEN 3) or SEV (ACURATE neo/TF). The selected endpoint combined early safety and clinical efficacy at 30 days. The ACURATE neo/TF SEV showed a higher rate of acute kidney failure and significant PVR but also better valvular areas and aortic gradients.
In the PORTICO IDE trial (2020),70 750 high- and extreme-risk patients were randomized to the Portico resheathable SEV system or any other commercially available valve. The former was non-inferior regarding death or stroke at one year of follow-up but was associated with higher rates of mortality and vascular complications at 30 days.
Finally, SOLVE-TAVI (2020)71 randomized 447 high-to-intermediate risk patients to an SEV (Evolut R) or a BEV (SAPIEN 3). The valve systems were equivalent regarding the primary endpoint of all-cause mortality, stroke, moderate to severe regurgitation or pacemaker implantation at 30-day follow-up.
Gaps and future challengesAs TAVI approaches 20 years of in-human experience, with favorable results in RCTs with patients of all risk strata, there are still important challenges to overcome. Firstly, patients with abnormal valve anatomies were excluded from the main trials. This is particularly important in younger, low-risk patients, in whom a bicuspid anatomy may be more prevalent. Secondly, there is still scope to decrease complications, such as those related to vascular access, conduction disturbances or significant PVR. TAVI-related complications are tending to decrease in successive generations of trials, arguably in part due to advances in valve structure and delivery systems and increased operator experience. Thirdly, large-scale studies directly comparing valves are lacking. The largest RCT comparing two TAVI systems is the REPRISE 3 trial, and only small trials compared older models of BEV and SEV. Fourthly, there have been no large-scale RCTs addressing the optimal management of coronary artery disease and severe AS. The implications of coronary artery bypass grafting and percutaneous coronary intervention on top of SAVR and TAVI, respectively, may be different, and should be addressed in an RCT. Fifthly, TAVI has only been compared with bioprosthetic surgical valves, hence the use of TAVI in younger patients, for whom a mechanical prosthesis would be suitable, has not been studied. Sixthly, the role of TAVI in asymptomatic patients with severe AS and decreased left ventricular function is unknown.72 Finally, there is a lack of data on long-term valve durability, which is particularly important when treating low-risk patients with a longer life expectancy.
ConclusionTAVI may be a recent approach to treat AS, but it tells a centuries-old story. Looking back at this roadmap, it is easy to see the increasing density of publications over the years, especially after the index TAVI procedure in 2002. More than 300 years after the first description of aortic stenosis, only a decade has passed since the first RCT in extreme-risk patients. The subsequent cascade of RCTs has progressively led to the current position of TAVI in the heart team discussion as a therapeutic option for the management of patients with aortic stenosis of all risk strata (Figure 1).
The roadmap to transcatheter aortic valve implantation. Above is the overall timeline of events since the first case descriptions of aortic stenosis. Below is the timeline of the major randomized clinical trials organized by valve type and patient risk, also displaying the longest follow-up study available to date. AS: aortic stenosis; BEV: balloon-expandable valve; HR: high risk; LR: low risk; MEV: mechanically-expandable valve; RCT: randomized clinical trial; SAVR: surgical aortic valve replacement; SEV: self-expanding valve; TAVI: transcatheter aortic valve implantation.
The authors have no conflicts of interest to declare.