Aortic pseudoaneurysms can be a potentially fatal, yet rare, complication of heart surgery. Surgery is indicated but is high risk during sternotomy. Therefore, careful planning is required. We report the case of a 57-year-old patient who underwent heart surgery twice in the past and who presented with an ascending aortic pseudoaneurysm. A successful repair of the pseudoaneurysm was performed under deep hypothermia, left ventricular apical venting, periods of circulatory arrest and endoaortic balloon occlusion.
Os pseudoaneurismas da aorta podem ser complicações potencialmente fatais, ainda que raras, da cirurgia cardíaca. A cirurgia é indicada mas tem alto risco durante a esternotomia. Desta forma, é necessário fazer um planeamento cuidadoso. Apresentamos o caso de um doente de 57 anos submetido a duas cirurgias cardíacas e que se apresentava com um pseudoaneurisma da aorta ascendente. A reparação bem-sucedida do pseudoaneurisma foi realizada sob hipotermia profunda, drenagem apical do ventrículo esquerdo, períodos de paragem circulatória e oclusão endoaórtica com balão.
Pseudoaneurysms are a rare complication after heart surgery. The development and expansion of aortic false aneurysms is often silent. Their evolution is unpredictable, and their management can be challenging. We report a case of a patient presenting with this complication following repeated heart surgery.
Case reportA 57-year-old male went to the emergency department of his local hospital several times in a two-month period with complaints of dyspnea and right thoracic pain. He denied cough, fever or other constitutional symptoms. The chest X-ray showed a mediastinal enlargement.
He had undergone aortic valve replacement with a mechanical bileaflet valve size 25 in 2011. In 2012, due to late prosthetic valve endocarditis, he had stentless bioprosthesis (Freestyle®) size 23 implanted using a subcoronary technique. Other comorbidities were type 2 diabetes, high blood pressure, dyslipidemia, and atrial fibrillation. In the physical examination, he had mild peripheral edema but no other alterations were found.
A computed tomography (CT) was subsequently performed. A large mediastinal collection, partially thrombosed, with dimensions of 105 mm×70 mm×150 mm was described, which appeared to have no cleavage plane with the ascending aorta or pulmonary artery. Our center was contacted for suspected ruptured aortic aneurysm and the patient was transferred to the intensive care unit.
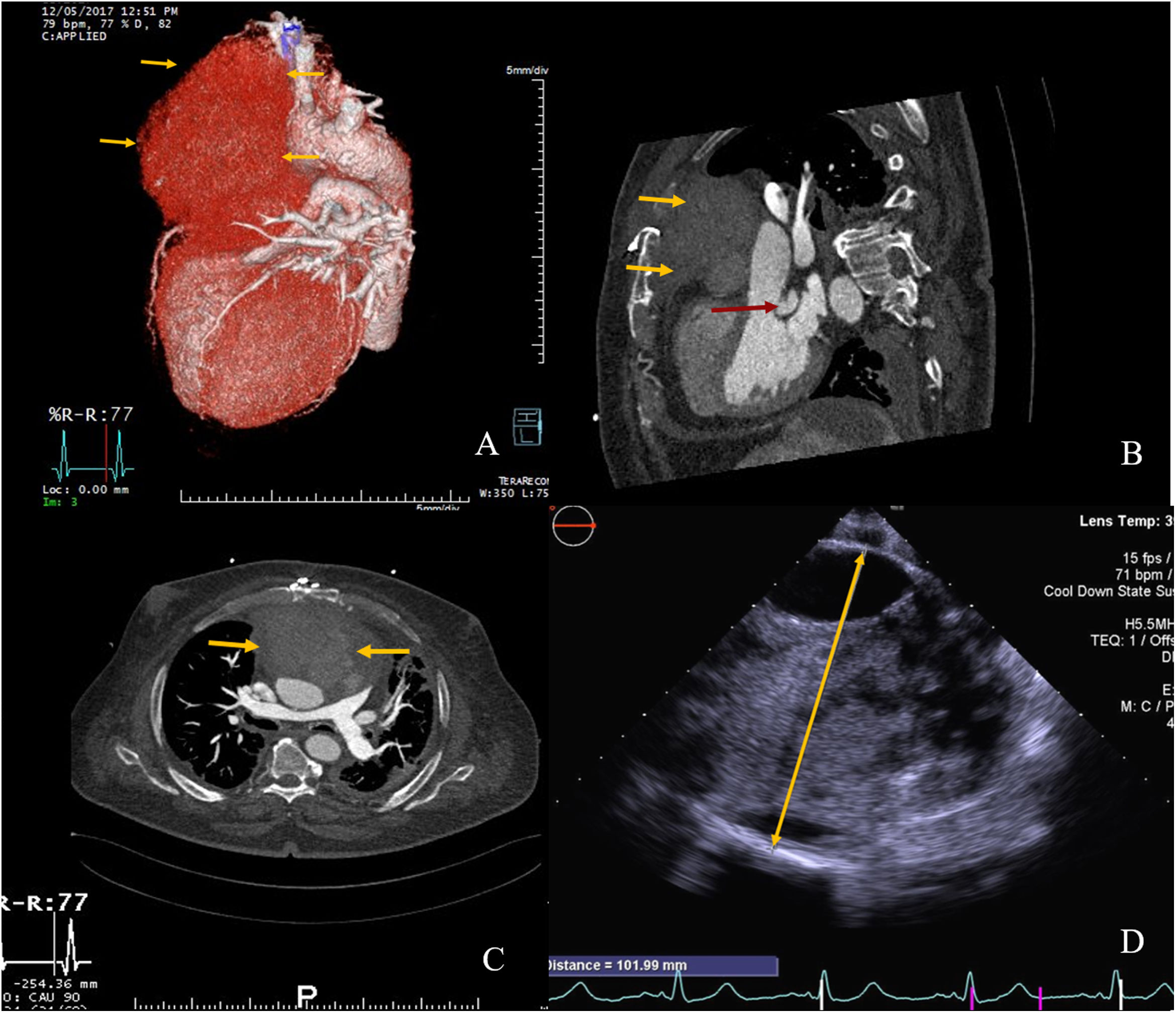
On arrival, he was hemodynamically stable and still under the effect of a new oral anticoagulant. Surgery was postponed enabling a washout of the anticoagulant. Meanwhile, labetalol was initiated for blood pressure control and a cardiac CT (Figure 1A–C) and a transthoracic echocardiogram were performed. The diagnosis was established as a large ascending aorta pseudoaneurysm which had already eroded the sternum. Also, a cavity posterior to the stentless valve with communication to the left ventricle outflow tract, which caused moderate periprosthetic leak, was described.
(A–C) Preoperative cardiac computed tomography (CCT). (A) Three-dimensional reconstruction showing the ascending aorta false aneurysm. (B) Two dimensional (2D) sagittal plane; yellow arrows: pseudoaneurysm eroding through the sternum; red arrow: periprosthetic posterior cavity. (C) 2D axial plane, yellow arrows: extrinsic main pulmonary artery compression due to pseudoaneurysm. (D) Intra-operative transoesophageal echocardiography (CT); yellow arrow: sizing of pseudoaneurysm (102 mm).
Surgery was carefully planned and began with the cannulation of both femoral and right subclavian arteries with straight tip, kink resistant, wire winding, cannulas 20 Fr and 14 Fr respectively, and of the femoral vein with a 28 Fr cannula of the same type. Cardiopulmonary bypass (CBP) started and deep hypothermia (20°C) was reached before chest opening. A vent was introduced in the apex of the left ventricle through a left anterior mini thoracotomy when ventricular fibrillation occurred. A balloon catheter was placed in the distal part of ascending aorta (guided by transoesophageal echocardiogram) and endoclamping of the aorta was performed only during sternotomy to avoid circulatory arrest. Antegrade cerebral perfusion was achieved through subclavian perfusion and by retrograde aortic flow through the arch vessels.
As expected, the sternotomy was complicated by a rupture of the large pseudoaneurysm, which contained fresh and organized thrombus, white pus and blood. The cavity was thoroughly washed, and parts of its wall were sent for pathological and microbiological analysis. The endoclamp balloon ruptured and so it was effective only for a few minutes. Antegrade cerebral perfusion had to be discontinued and further brain protection was achieved pharmacologically and with hypothermia during the short periods of circulatory arrest. No cardioplegia was given at any time, due to a short period of circulatory arrest in deep hypothermia.
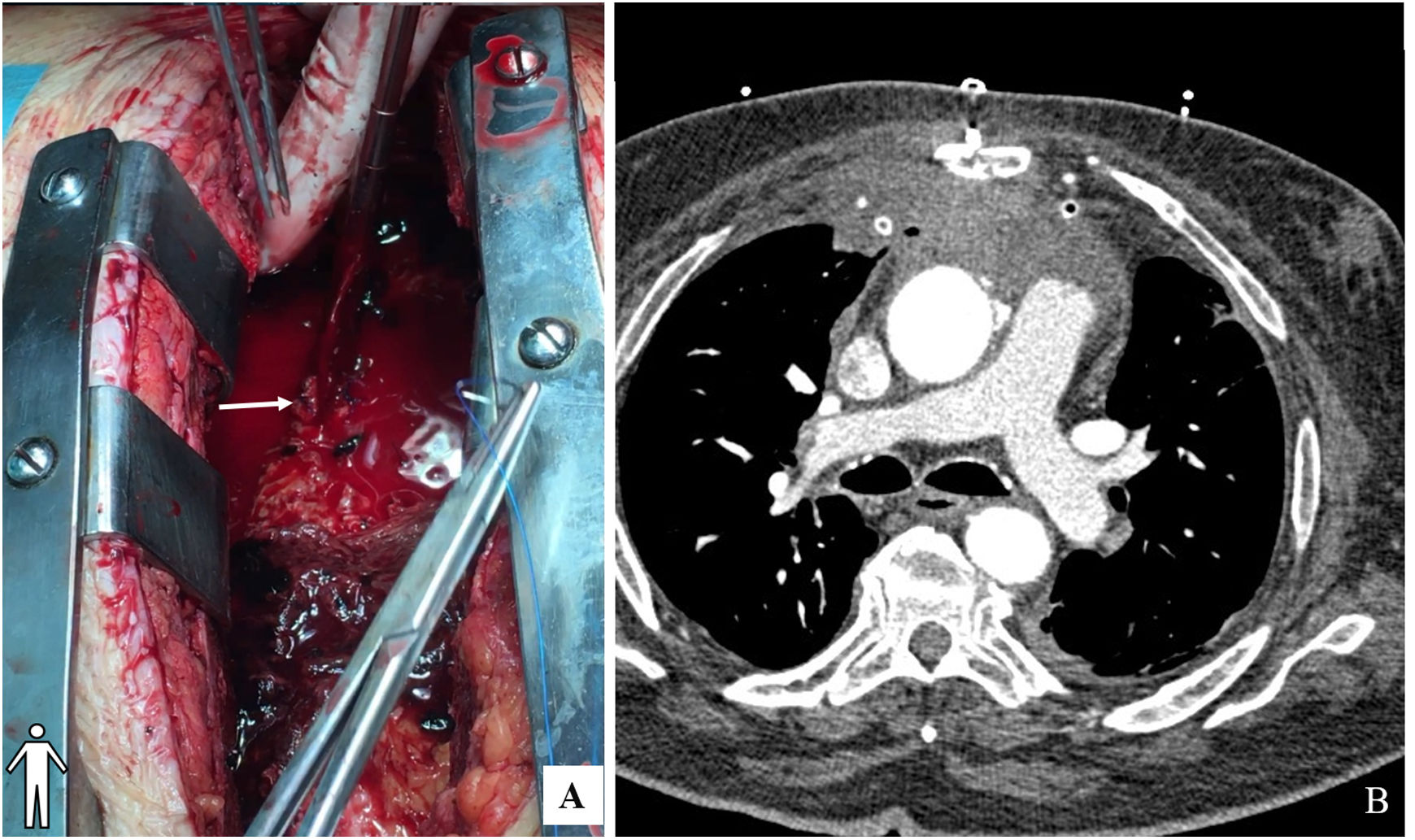
Two bleeding points were identified in the aortic wall (aortic cannulation and antegrade cardioplegia sites) and the rest of the aortic wall was reasonably preserved and had a normal diameter. The hypothesis of local infection and the complexity of a complete replacement of the aorta, of the previous prosthetic valve and eventually coronary reimplantation in a prosthetic conduit was considered too high risk a procedure. Two bleeding points were corrected with direct buttressed sutures (Teflon pledgets) (Figure 2A). Short periods of low flow and circulatory arrest were used to enable visualization and correction of the bleeding points. CBP was discontinued after warming and the sternum was closed. Total CPB time and circulatory arrest was 194 minutes and 7 minutes, respectively. TOE (Figure 1D and video 1) was performed intra-operatively and regarding the pseudoaneurysm, the surgical outcome was positive.
Post-operatively the patient progressed well. Empirical antibiotics (vancomycin, gentamicin, and rifampicin) were initiated. He was extubated on the second postoperative day and was transferred to the ward on the fourth day. Postoperative transthoracic echocardiogram and chest CT (Figure 2B) demonstrated a good surgical result. Microbiological and pathological analysis were negative for active infection, so antibiotics were discontinued after 21 days. The patient was transferred to this local hospital on day 13. TOE performed one month after surgery still described a periprosthetic leak in relation to a posterior cavity in the aortic root. A few months later, the patient underwent transcatheter closure of the perivalvular leak with good result.
DiscussionPseudoaneurysms occur when there is a disruption of one or more, but not all, layers of the wall of a vessel, that is contained by the remaining layers or surrounding tissues. It is distinguishable from a true aneurysm, in which the vessel wall remains intact.1 They are a rare (less than 0.5% of all heart surgical cases) but potentially fatal complication of heart surgery.2,3
Pseudoaneurysms may occur months to years after surgery.1,4 Predisposing factors include aortic dissection, infection, connective tissue disorders and chronic hypertension.2,3
Postoperative pseudoaneurysms of the ascending aorta usually occur at sites of aortic wall disruption, such as suture lines. In a review of 31 cases of postoperative pseudoaneurysms, Sullivan et al.,1 described the aortic cannulation site and aortotomy as the most frequent sites of origin. The origin of this patient's false aneurysm was previous cannulation site (aortic cannula/antegrade cardioplegia). He had hypertension and a past episode of endocarditis as risk factors.
Pseudoaneurysms are usually asymptomatic at first, but later various symptoms may arise due to compression of surrounding structures.1,2 This patient complained of chest pain and dyspnea, probably due to compression of adjacent structures.
Imaging exams allow perception of location, extension, neck size, and proximity to coronaries.3 Mediastinal widening may be seen in chest radiography, but the diagnosis is most accurate with a CT.1 Echocardiography, angiography and MRI may also be useful.2 In this patient, imaging tests were essential for diagnosis and planning surgery.
The potential risk of rupture and pseudoaneurysm enlargement increase with time.2 Surgical intervention is therefore absolutely indicated2 and should be planned considering the high risk of pseudoaneurysm rupture during sternal opening with resulting exsanguination. Brain protection from ischemia should also not be overlooked.3 CBP can be initiated with femoral or other peripheral vessels cannulation and hypothermia performed to allow circulatory arrest when needed.1 Suction and decompression of the left ventricle is advised in cases of aortic regurgitation as soon as the heart ceases to eject, to avoid subendocardial ischemia and ventricular dysfunction. In this case, despite an additional incision, the LV apex was promptly accessed. Balloon occlusion of the ascending aorta with retrograde perfusion through the femoral artery allowed us to minimize the circulatory arrest time. However, the balloon may be cumbersome to use and it must not occlude the supra aortic vessels. Direct cannulation and cross-clamping of both carotid arteries with selective cerebral perfusion with cold blood is also an option.4 The choice of subclavian and femoral cannulation allows for antegrade flow in the aortic arch with low cerebral perfusion pressure. Even if the position of the endoclamp is not perfect (e.g., occluding the bracheo-cephalic trunk), antegrade cerebral perfusion can occur by either head vessels. The successful exclusion of pseudoaneurysm percutaneously by using Amplatzer muscular ventricular septal defect occluders has been recently described.5 However, in this case, considering the size of the pseudoaneurysm, the mediastinal compression and the sternum erosion, it was not considered a good option.
Mortality ranges from 29 to 46%, and exsanguination due to rupture of the pseudoaneurysm the most frequent cause of death.3 This case had a good outcome. In this case we initiated CBP using peripheral cannulation and periods of circulatory arrest when needed under deep hypothermia. The patient suffered no neurological lesions nor peripheral ischemia. However, if sternotomy had been attempted before CPB and hypothermia were established, the result would have been almost certainly fatal.
Conflicts of interestThe authors have no conflicts of interest to declare.