The neutrophil-to-lymphocyte ratio (NLR) is established as a reliable marker of systemic inflammation. Low-grade inflammation has a key role in the pathogenesis and progression of hypertension (HTN). Blood pressure (BP) load, defined as the percentage of abnormally elevated BP readings, is a good marker of HTN severity. We aimed to evaluate the relationship between HTN severity and NLR using averaged ambulatory BP readings and BP load.
MethodsA total of 300 patients with untreated essential HTN were included in this cross-sectional study. Patients were divided into quartiles according to NLR values (first: <1.55; second: 1.55-1.92; third: 1.92-2.48; and fourth: >2.48). Averaged ambulatory BP values and BP load were assessed for each quartile.
ResultsIn the interquartile evaluation there were no differences between quartiles in terms of baseline demographic, clinical and echocardiographic characteristics (p>0.05). Daytime systolic BP (SBP), 24-hour diastolic BP (DBP), daytime DBP, daytime SBP load, 24-hour DBP load and daytime DBP load were found to be significantly higher in the upper two quartiles (p<0.05 for all). In correlation analysis, log NLR values were found to be positively correlated with 24-hour SBP, DBP, SBP load and DBP load (Pearson coefficients of 0.194, 0.197, 0.157 and 0.181, respectively; p<0.01 for all). In multivariate analysis, log NLR had an independent association with 24-hour SBP and DBP and 24-hour SBP and DBP load.
ConclusionThis study showed for the first time that increased NLR is independently associated with HTN severity in untreated essential HTN patients.
A relação neutrófilos/linfócitos (RNL) foi estabelecida como um marcador inflamatório sistémico fiável. Uma inflamação de baixo grau tem um papel fundamental na patogénese e na progressão da hipertensão (HT). A carga da pressão arterial (PA) definida como a percentagem de leituras da PA anormalmente elevadas é um bom marcador da gravidade da PA. O objetivo deste estudo é avaliar a relação entre a gravidade da PA e a RNL pela utilização da média de leituras ambulatórias da PA e da carga da PA.
MétodosUm total de 300 doentes, com HT essencial não tratada, foram incluídos neste estudo transversal. Os doentes foram divididos em quartis, de acordo com os valores da RNL (primeiro <1,55; segundo 1,55-1,92; terceiro 1,92-2,48 e o quarto > 2,48). Os valores médios da PA em ambulatório e a carga da PA foram avaliados para cada quartil.
ResultadosNa avaliação interquartis, não houve diferença entre quartis nas características demográficas, clínicas e ecocardiográficas basais (p > 0,05). A PA sistólica diurna (PAS), a PA diastólica (PAD) a 24 horas, a PAD diurna, a carga da PAS diurna, a PAD a 24 horas e a PAD diurna foram significativamente mais elevadas nos dois quartis superiores (p < 0,05 para todos). Na análise de correlação, os valores do logaritmo de RNL foram positivamente correlacionados com a PAS a 24 horas, com a PAD, com a PAS carga e com a PAD carga (coeficientes de Pearson de 0,194, 0,197, 0,157 e 0,181, respetivamente; p<0,01 para todos). Na análise multivariada, os valores do logaritmo da RNL mostraram uma associação independente com a PAS-PAD a 24 horas e com a carga da PAS-PAD a 24 horas.
ConclusãoEste estudo mostrou pela primeira vez que o aumento da RNL está independentemente associado à gravidade da PA nos doentes com HT essencial não tratada.
Hypertension (HTN) is a common condition that results in damage to important target organs including the heart, kidney and brain.1,2 Low-grade inflammation has a key role in its pathogenesis and progression.3 Inflammatory processes are assumed to have particularly significant involvement in vascular remodeling of resistance arteries.4 Increasing blood pressure (BP) may progressively cause a pro-inflammatory response and thus establish a vicious cycle. Furthermore, HTN is a significant risk factor in inflammatory conditions such as atherosclerosis.5
The relationship between various cardiovascular conditions and inflammation has been investigated in previous studies.6 Elevated C-reactive protein (CRP), vascular (VCAM-1) and intercellular (ICAM-1) adhesion molecules, monocyte chemoattractant protein-1 (MCP) and plasminogen activator inhibitor-1 (PAI-1) are some of the inflammatory molecules that are increased in HTN.7,8 Along with these well-known inflammatory markers, recent studies have shown the neutrophil-to-lymphocyte ratio (NLR) to be a reliable marker of systemic inflammation.9 It has been shown to have prognostic significance in various conditions such as coronary artery disease,10,11 malignancy,12 heart failure,13 and cerebral and peripheral artery disease.14,15 Since HTN is considered a significant risk factor in most of these conditions, the relationship between NLR and HTN has also been thoroughly investigated.16 NLR is a good predictor in high-risk conditions such as resistant HTN and non-dipper HTN.17,18
Ambulatory blood pressure monitoring (ABPM) is an important tool that is frequently used by clinicians in daily practice to guide treatment and help to identify conditions such as white-coat and masked HTN.19 It is known that ABPM is a better predictor of target organ damage and cardiovascular endpoints than office BP.20 However, it may have limitations in patients with ‘high-normal’ BP.21 In view of this limitation, some authors suggest that BP load, defined as the percentage of abnormally elevated BP readings, is a better predictor.22,23
As far as we know, there are few data on the evaluation of severity of the inflammatory response in HTN using NLR, which is a simple and inexpensive method. Thus, in our study we aimed to evaluate the relationship between HTN severity and NLR using averaged ambulatory BP readings and BP load.
MethodsStudy populationThis cross-sectional study included 300 consecutive patients with newly diagnosed essential hypertension by 24-hour ABPM using a validated device between December 2014 and December 2015. All patients had untreated essential HTN, defined as office BP of ≥140/90 mmHg (the mean of ≥2 valid readings measured on at least two visits). Patients with the following criteria were excluded from the study: current use of antihypertensive drugs, acute or chronic infectious conditions, leukocytosis (white blood cell count >12.0×103/mm3), history of malignancy, chronic renal disease (estimated glomerular filtration rate <60 ml/min/1.73 m2), elevated hepatic enzymes (bilirubin >2 upper limit of normal [ULN] or AST/ALT/ALP >3 ULN), diagnosis of secondary hypertension or white coat hypertension, moderate to severe valvular disease, symptomatic cardiac failure or prosthetic valve, history of coronary artery disease, peripheral artery disease or stroke, atrial fibrillation, thromboembolic disorders, history of hematological disease and consumption of drugs that may affect NLR.
Demographic, clinical, and laboratory parameters including age, gender, diabetes, hyperlipidemia, smoking status, body mass index (BMI), fasting blood glucose level, serum creatinine level, fasting lipid profile, and complete blood count parameters were recorded in all patients.
Informed consent was obtained from each patient before enrollment. The study was approved by the institutional ethics committee and performed in accordance with the Helsinki Declaration.
Laboratory testsA complete blood count analysis was performed using an LH series analyzer (Beckman Coulter Inc., Hialeah, FL). NLR was calculated as the ratio of neutrophil count to lymphocyte count in admission samples. Biochemistry analysis was performed using standard tests on samples obtained in fasting conditions.
All transthoracic echocardiography examinations were performed using a Philips iE33 xMATRIX system with a 2.5/3.5 MHz transducer (Philips Electronics, The Netherlands) by an experienced echocardiographer who was blinded to the patients included in the study. Left ventricular ejection fraction (LVEF) and left ventricular mass index (LVMI) were calculated as recommended by the current guidelines.24 An electrocardiogram was obtained for all patients.
Blood pressure measurementTwenty-four-hour ABPM readings (Oscar 2 oscillometric monitor, SunTech Medical Inc., Morrisville, NC) were assessed for all patients. Appropriate cuff sizes were selected by trained nurses. Measurements were taken at 15-min intervals during the day and at 30-min intervals at night. Patients with fewer than 80% valid measurements were excluded. Patients with mean 24-hour systolic BP (SBP) ≥130 mmHg and/or diastolic BP (DBP) ≥80 mmHg or mean daytime SBP ≥135 mmHg and/or DBP ≥85 mmHg were diagnosed as hypertensive.19 A non-dipper pattern was defined as a decrease of <10% in SBP between daytime (7:00 am to 11:00 pm) and nighttime (11:00 pm to 7:00 am). Mean 24-hour, daytime, and nighttime SBP and DBP were calculated for each patient using the hourly averages of ambulatory BP recordings. BP load and BP variability were recorded as additional data. BP load was defined as the percentage of values reaching or exceeding 135 mmHg SBP or 85 mmHg DBP daytime and 120 mmHg SBP or 70 mmHg DBP nighttime on 24-hour BP readings. 24-hour BP load was also calculated based on these cut-off values. For a more reliable evaluation of BP variability, we also performed an additional analysis using the weighted mean of daytime and nighttime standard deviation for both SBP and DBP, as previously described.25
Statistical analysisStatistical analyses were performed using SPSS software (version 21.0; SPSS, Chicago, IL). Continuous data were presented as medians with interquartile range or mean ± SD for non-normally and normally distributed variables, respectively. The Shapiro-Wilk test was used to test the distribution pattern and logarithmic conversion was performed for non-normally distributed variables. The study population was categorized into quartiles based on NLR levels. Comparisons between groups were carried out by Kruskal-Wallis tests or analysis of variance, as appropriate. Differences between groups were revealed using Dunn's procedure for data without normal distribution and Bonferroni's multiple comparison post hoc test for data with normal distribution. Categorical variables were summarized as percentages and compared with the chi-square test. Correlations between variables were investigated by the Pearson correlation coefficient. Age, gender, diabetes, hyperlipidemia, smoking status, BMI, serum creatinine, hemoglobin, fasting glucose, white blood cell count, platelet count, log NLR, total cholesterol, low-density lipoprotein, high-density lipoprotein cholesterol, triglycerides, aspirin medication, LVEF and LVMI were tested by univariate linear regression analysis. Multivariate regression analysis including all univariate correlates (p<0.1) was used to identify predictors for 24-hour systolic and diastolic BP and BP load. For all tests, statistical significance was accepted as a p value <0.05.
ResultsThree hundred hypertensive patients (47% male) were evaluated. The mean age of the population was 48.5±14 years. Patients were divided into quartiles according to NLR values (first: <1.55; second: 1.55-1.92; third: 1.92-2.48; and fourth: >2.48). There were no differences between quartiles in terms of basal demographics, cardiovascular risk factors or echocardiographic parameters (Table 1). Baseline laboratory findings are presented in Table 2. Fasting blood glucose, serum creatinine, hemoglobin, platelet count, and lipid profile were not different across NLR quartiles. White blood cell and neutrophil counts were significantly higher in upper quartiles of NLR. There was also a statistically significant trend towards lower lymphocyte count in patients in the fourth quartile.
Baseline characteristics of patients by neutrophil-to-lymphocyte ratio quartiles.
| All (n=300) | 1st quartile (n=75) | 2nd quartile (n=75) | 3rd quartile (n=75) | 4th quartile (n=75) | pa | |
|---|---|---|---|---|---|---|
| Age, years | 48.5±14 | 47.7±14 | 48.5±13 | 47.7±15 | 49.9±14 | 0.776 |
| Male, n (%) | 141 (47) | 37 (49) | 40 (53) | 32 (43) | 32 (43) | 0.457 |
| BMI, kg/m2 | 22.4 (21.1-23.7) | 22.8 (21.3-23.7) | 22.1 (20.9-23.6) | 22.3 (21.1-23.7) | 22.1 (21.1-23.7) | 0.543 |
| Diabetes, n (%) | 41 (14) | 7 (9) | 12 (16) | 9 (12) | 13 (17) | 0.479 |
| Hyperlipidemia, n (%) | 21 (7) | 1 (1) | 8 (11) | 8 (11) | 4 (5) | 0.071 |
| Smoking, n (%) | 36 (12) | 8 (11) | 12 (16) | 11 (15) | 5 (7) | 0.290 |
| Aspirin therapy, n (%) | 4 (1) | 1 (1) | 0 | 1 (1) | 2 (3) | 0.568 |
| Echocardiographic characteristics | ||||||
| IVS, mm | 10.4±1.5 | 10.2±1.6 | 10.5±1.5 | 10.2±1.4 | 10.5±1.6 | 0.578 |
| Posterior wall, mm | 9.9±1.3 | 9.7±1.2 | 10.0±1.3 | 9.8±1.2 | 9.9±1.5 | 0.666 |
| LVEDD, mm | 46.1±3.5 | 46.4±3.7 | 45.8±2.7 | 46.2±3.7 | 46.2±3.8 | 0.822 |
| LVEF, % | 61.9±2.9 | 61.7±3.0 | 62.1±2.6 | 61.6±3.0 | 62.3± 3.2 | 0.494 |
| LVMI, g/m2 | 93.3±19.4 | 92.6±19.4 | 94.0±19.3 | 92.3±18.7 | 94.3±20.6 | 0.897 |
BMI: body mass index; IVS: interventricular septum; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVMI: left ventricular mass index; NLR: neutrophil-to-lymphocyte ratio. Data are expressed as mean ± standard deviation, median with interquartile range, or percentage frequency, as appropriate. NLR quartiles: first: 0.74-1.55; second: 1.55-1.92; third: 1.92-2.48; fourth: 2.48-6.64.
Laboratory parameters of patients by neutrophil-to-lymphocyte ratio quartiles.
| All (n=300) | 1st quartile (n=75) | 2nd quartile (n=75) | 3rd quartile (n=75) | 4th quartile (n=75) | pa | |
|---|---|---|---|---|---|---|
| Fasting blood glucose, mg/dl | 90 (81-102) | 89 (81-99) | 90 (82-104) | 90 (82-99) | 91 (81-106) | 0.914 |
| Serum creatinine, mg/dl | 0.85 (0.72-1.0) | 0.83 (0.70-1.0) | 0.86 (0.74-0.97) | 0.81 (0.70-0.98) | 0.88 (0.73-1.03) | 0.679 |
| Hemoglobin, g/dl | 14.3±1.5 | 14.4±1.4 | 14.4±1.5 | 14.4±1.5 | 14.1±1.6 | 0.374 |
| WBC, 103/mm3 | 7.8±1.7 | 6.8±1.5 | 7.8±1.4c | 7.9±1.5 | 8.6±2.0b | <0.001 |
| Neutrophils, 103/mm3 | 4.7±1.4 | 3.4±0.8 | 4.3±0.7d | 4.9±1.0b | 5.9±1.5d | <0.001 |
| Lymphocytes, 103/mm3 | 2.3±0.6 | 2.7±0.6 | 2.5±0.4 | 2.2±0.4c | 1.9±0.4d | <0.001 |
| NLR | 2.09±0.83 | 1.27±0.18 | 1.71±0.10 | 2.19±0.16 | 3.2±0.80 | - |
| Platelets, 103/mm3 | 265±67 | 251±64 | 263±62 | 269±64 | 279±75 | 0.075 |
| Total cholesterol, mg/dl | 202±40 | 205±34 | 206±38 | 201±45 | 198±43 | 0.582 |
| HDL cholesterol, mg/dl | 46 (40-54) | 45 (40-56) | 47 (40-55) | 46 (39-55) | 45 (40-51) | 0.540 |
| LDL cholesterol, mg/dl | 143±35 | 144±32 | 148±35 | 142±37 | 140±35 | 0.611 |
| Triglycerides, mg/dl | 143 (94-215) | 145 (90-214) | 159 (102-217) | 135 (91-224) | 126 (91-183) | 0.247 |
HDL: high-density lipoprotein; LDL: low-density lipoprotein; NLR: neutrophil-to-lymphocyte ratio; WBC: white blood cell count. Data are expressed as mean ± standard deviation or median with interquartile range, as appropriate. NLR quartiles: first: 0.74-1.55; second: 1.55-1.92; third: 1.92-2.48; fourth: 2.48-6.64.
Patients’ ABPM data are shown in Table 3. In the interquartile evaluation, daytime SBP, 24-hour DBP, daytime DBP, daytime SBP load, 24-hour DBP load and daytime DBP load were found to be significantly higher in the upper two quartiles (p<0.05 for all). The difference was more noticeable in DBP data. Dipping status and BP variability data were not significantly different between quartiles (p>0.05).
Comparison of ambulatory blood pressure monitoring data across neutrophil-to-lymphocyte ratio quartiles.
| All (n=300) | 1st quartile (n=75) | 2nd quartile (n=75) | 3rd quartile (n=75) | 4th quartile (n=75) | pa | |
|---|---|---|---|---|---|---|
| 24-h SBP, mmHg | 139.7±12.0 | 137.0±11.2 | 139.0±10.9 | 140.4±11.0 | 142.2±14.0 | 0.09 |
| Daytime SBP, mmHg | 142.4±11.8 | 139.7±10.6 | 141.5±11.3 | 143.5±11.1 | 144.7±13.6 | 0.048 |
| Nighttime SBP, mmHg | 127 (118-137) | 126 (117-135) | 125 (118-133) | 127 (118-139) | 132 (117-140) | 0.295 |
| 24-h DBP, mmHg | 83.6±9.0 | 80.7±8.4 | 83.0±8.8 | 84.6±8.6b | 85.8±9.5c | 0.003 |
| Daytime DBP, mmHg | 85.7±9.0 | 82.8±8.3 | 84.9±8.9 | 87.1±8.7c | 87.9±9.5c | 0.001 |
| Nighttime DBP, mmHg | 74 (68-82) | 71 (63-80) | 73 (68-80) | 74 (68-82) | 77 (70-85) | 0.059 |
| Non-dippers, n (%) | 151 (51) | 40 (54) | 30 (40) | 37 (49) | 44 (59) | 0.126 |
| SD of 24-h SBP, mmHg | 15.6 (13.2-18.5) | 15.2 (12.6-18.0) | 16.1 (14.4-18.8) | 15.1 (12.4-18.9) | 15.8 (13.0-18.6) | 0.170 |
| SD of 24-h DBP, mmHg | 11.4 (9.5-13.9) | 11.2 (9.2-13.8) | 11.9 (10.6-14.0) | 11.1 (9.3-13.7) | 11.1 (9.1-14.1) | 0.374 |
| wSD of SBP | 13.9 (11.7-16.3) | 13.4 (11.5-15.6) | 14.8 (13.0-16.9) | 13.2 (11.6-17.0) | 14.1 (11.5-15.4) | 0.152 |
| wSD of DBP | 10.2 (8.6-12.3) | 9.9 (8.1-11.5) | 10.4 (9.1-12.4) | 10.1 (8.6-12.8) | 10.0 (8.3-12.2) | 0.469 |
| 24-h pulse pressure | 56.1±9.0 | 56.3±9.2 | 55.8±9.0 | 56.0±8.0 | 56.4±9.8 | 0.993 |
| 24-h SBP load, (%) | 60.2±23.4 | 54.8±23.3 | 58.8±21.7 | 63.2±22.1 | 63.8±25.6 | 0.084 |
| Daytime SBP load, (%) | 59.1±24.1 | 53.3±23.8 | 57.4±22.2 | 62.9±23.1 | 62.6±26.3 | 0.045 |
| Nighttime SBP load, (%) | 71 (43-100) | 57 (32-90) | 71 (43-86) | 71 (43-100) | 83 (43-100) | 0.454 |
| 24-h DBP load, (%) | 50.9±26.3 | 42.2±26.2 | 48.6±23.9 | 55.8±26.5c | 56.8±26.2c | 0.001 |
| Daytime DBP load, (%) | 48.4±27.6 | 39.5±26.4 | 45.6±25.5 | 54.5±28.3c | 54.0±27.7c | 0.002 |
| Nighttime DBP load, (%) | 60 (33-86) | 57 (26-86) | 57 (33-86) | 67 (43-86) | 71 (43-100) | 0.087 |
DBP: diastolic blood pressure; NLR: neutrophil-to-lymphocyte ratio; SBP: systolic blood pressure; SD: standard deviation; wSD: weighted standard deviation. Data are expressed as mean ± standard deviation (SD), median with interquartile range, or percentage frequency, as appropriate. Bold values indicate statistical significance. NLR quartiles: first: 0.74-1.55; second: 1.55-1.92; third: 1.92-2.48; fourth: 2.48-6.64.
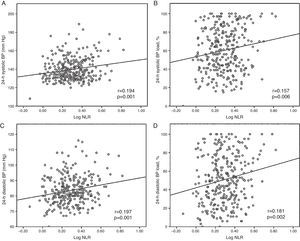
Subsequently, the relationship between mean BP and BP load values and NLR was evaluated. A positive correlation between log NLR and daytime, nighttime and 24-hour BP and BP load values was identified (p<0.05 for all). Correlations of 24-hour systolic BP (r=0.194; 95% confidence interval [CI]: 0.074-0.320, p=0.001) and 24-hour diastolic BP (r=0.197; 95% CI: 0.076-0.307, p=0.001), 24-hour systolic BP load (r=0.157; 95% CI: 0.032-0.278, p=0.006) and 24-hour diastolic BP load (r=0.181; 95% CI: 0.054-0.295, p=0.002) with log NLR are shown in Figure 1. Additionally, daytime SBP load (r=0.150, 95% CI: 0.025-0.265, p=0.009), nighttime SBP load (r=0.129, 95% CI: 0.011-0.247, p=0.026), daytime DBP load (r=0.160, 95% CI: 0.040-0.273, p=0.005) and nighttime DBP load (r=0.173, 95% CI: 0.049-0.289, p=0.003) were positively correlated with log NLR. Similarly, a significant positive correlation was observed between mean daytime SBP (r=0.173, 95% CI: 0.055-0.292, p=0.003), nighttime SBP (r=0.206, 95% CI: 0.091-0.332, p< 0.001), daytime DBP (r=0.170, 95% CI: 0.059-0.282, p=0.003) and nighttime DBP (r=0.214, 95% CI: 0.096-0.331, p<0.001) and log NLR values.
After construction of multiple linear regression models with the four ABPM components associated with log NLR levels as independent variables, log NLR values maintained an independent association with 24-hour SBP, 24-hour DBP, 24-hour SBP load (Table 4) and 24-hour DBP load (Table 5). When 24-hour SBP was taken as a continuous variable, multivariate linear regression analysis revealed that 24-hour SBP was correlated with fasting blood glucose (β=0.160, 95% CI: 0.016-0.088, p=0.005), female gender (β=-0.114, 95% CI: -5.409-0.078, p=0.044), LVMI (β=0.194, 95% CI: 0.049-0.191, p=0.001) and log NLR (β=0.208 95% CI: 7.881-23.975, p<0.001). Similarly, multivariate linear regression analysis demonstrated significant associations between age (β=-0.197, 95% CI: -0.197-0.047, p=0.002), LVEF (β=-0.162, 95% CI: -0.837-0.149, p=0.005), log NLR (β=0.216, 95% CI: 6.119-18.741, p<0.001) and 24-hour DBP.
Univariate and multivariate linear regression models for 24-hour systolic blood pressure load.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| Female | -0.113 | -10.6-0.01 | 0.05 | -0.04 | -8.257-4.523 | 0.566 |
| Age | 0.142 | 0.047-0.410 | 0.014 | 0.063 | -0.088-0.292 | 0.293 |
| BMI | 0.102 | -0.179-3.431 | 0.077 | 0.097 | -0.188-3.266 | 0.08 |
| Fasting blood glucose | 0.193 | 0.052-0.194 | 0.001 | 0.153 | 0.024-0.170 | 0.009 |
| Hemoglobin | 0.096 | -0.274-3.357 | 0.096 | 0.121 | -0.199-4.077 | 0.075 |
| LVEF | -0.104 | -1.713-0.078 | 0.073 | -0.081 | -1.517-0.238 | 0.153 |
| LVMI | 0.241 | 0.156-0.423 | <0.001 | 0.151 | 0.04-0.325 | 0.012 |
| Log NLR | 0.157 | 6.643-40.293 | 0.006 | 0.174 | 9.818-42.135 | 0.002 |
BMI: body mass index; CI: confidence interval; LVEF: left ventricular ejection fraction; LVMI: left ventricular mass index; NLR: neutrophil-to-lymphocyte ratio. Bold values indicate statistical significance.
Univariate and multivariate linear regression models for 24-hour diastolic blood pressure load.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| Age | -0.186 | -0.539-0.134 | 0.001 | -0.178 | -0.525-0.118 | 0.002 |
| Creatinine | 0.099 | -0.074-1.093 | 0.087 | 0.077 | -0.176-0.964 | 0.174 |
| HDL cholesterol | -0.099 | -0.545-0.038 | 0.088 | -0.043 | -0.402-0.179 | 0.451 |
| Log NLR | 0.181 | 11.448-49.088 | 0.002 | 0.177 | 11.09-48.214 | 0.002 |
CI: confidence interval; HDL: high-density lipoprotein; NLR: neutrophil-to-lymphocyte ratio. Bold values indicate statistical significance.
The results of our study show that in patients with untreated HTN, admission NLR values and ambulatory BP and BP load measurements were correlated. To our knowledge, this is the first study in the literature demonstrating such a correlation between NLR and BP severity.
Elevated NLR values are a predictor of high cardiovascular risk26 and are correlated with inflammatory activity and the severity of numerous diseases.27,28 Increased renin-angiotensin-aldosterone system activity and CRP-mediated inflammation are involved in the inflammatory response observed in hypertensive patients, and activation of peroxisome proliferator-activated receptors may protect against these effects.4 Angiotensin II triggers a series of inflammatory processes resulting in vascular remodeling and injury via endothelin-1 and NAD(P)H oxidase.29 Other than the vascular remodeling resulting from chronic HTN, there is no other evidence indicating whether low-grade inflammation level is reflected in short-term BP values. However, structural vascular alterations occurring during chronic HTN, along with some functional mechanisms, may cause acute BP alterations and inflammation.30 In previous studies it was shown that high-sensitivity CRP and leukocyte count were elevated in patients experiencing acute BP increase in conditions such as hypertensive crisis.31 Shear stress due to high BP levels may lead to an increase in inflammation in the short term by increased expression of adhesion molecules, endothelial dysfunction and NO-related mechanisms.32 It is known that ICAM-1 and interleukin-6 increase with increasing BP.33 In our study also, patients with higher NLR were found to have higher ambulatory BP measurements, particularly for DBP. Like mean BP values, the relationship of BP load with higher NLR values suggests that the inflammation observed in these patients is associated with short-term barotrauma. A higher BP load means that target organs are exposed to higher pressure for a longer duration, even if mean BP is normal. Additionally, patients with increased BP load are subject to overloading of the cardiovascular system, with consequent negative impact on related structures. Higher BP load may promote an inflammatory response to HTN. Recent studies reporting an association between BP load and target organ damage have shown that this relationship is not independent of 24-hour BP levels.34 On the contrary, Wang et al. have shown that BP load is an independent predictor of target organ damage in patients with chronic renal disease.23 Higher BP load (‘baric impact’) may cause endothelial dysfunction associated with vasopressor effects and thus may increase inflammation.35
As a probable predictor of BP severity, NLR has been the subject of antihypertensive therapy studies. Antihypertensive drugs may lead to a decrease in NLR, by both BP-lowering and anti-inflammatory effects.36,37 In previous studies non-dipping status and increased BP variability were associated with higher NLR.38,39 In our study, the lack of difference in BP variability and non-dipper status between groups made the relationship between NLR and BP/BP load clearer. Furthermore, increased pulse pressure is considered a significant marker of chronic inflammation and arterial stiffness.40 In a smaller cohort including normotensives NLR was reportedly correlated with 24-hour SBP and night-time SBP.41 In the same study, pulse pressure was also correlated positively with NLR. In our study, mean pulse pressure did not differ between quartiles and therefore did not contribute significantly to the results. Interestingly, Gang and Yanyan showed that NLR was increased in patients suffering from HTN with hyperhomocysteinemia and was positively correlated with homocysteine levels but not with BP.42 In their study, they also found no difference in NLR values between hypertensive and normotensive groups. A single office BP measurement and lack of data on antihypertensive medication could be responsible for their results.
In the present study, we found a weak correlation between ABPM data and log NLR values. There are several possible reasons for this: 24-hour ABPM provides short-term data and has limited reproducibility, which makes it difficult to clarify the severity of HTN. In addition, the selection of a relatively low-risk cohort of hypertensive patients may have meant they had only a low-grade inflammatory response, which might have weakened the correlation between NLR and severity of HTN. Nevertheless, despite other confounding factors such as the unknown chronicity of HTN and underlying subclinical end-organ damage, the available data suggest that higher NLR is associated with higher BP and BP load. Thus, our study may function as a hypothesis-generating study and lead to further research.
Our study has some limitations. Firstly, its cross-sectional design precludes drawing conclusions about causal relationships between NLR values and short-term ABPM results. Since ABPM was performed only once in each patient, the reproducibility of ambulatory BP measurements is another limitation. We think that the use of BP load as an outcome is also a limitation because its importance in cardiovascular risk stratification is debatable. In addition, it would have been better to have evaluated leukocyte activation markers, pro-inflammatory cytokines and oxidative stress markers. Exclusion of patients with severe complications of HTN from the present study prevents us from generalizing our results to the whole hypertensive patient population.
ConclusionsWe showed for the first time a significant correlation between high BP load and NLR. Further large-scale studies are needed to assess whether NLR can be used to predict HTN severity and to guide therapy in patients with HTN.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.