Heart failure (HF) is among the leading causes of morbidity and mortality worldwide. Several conditions trigger left ventricular chronic pressure or volume overload, hypertrophy, systolic and diastolic dysfunction, leading to cardiac remodeling and a rapid progression toward HF. Therapeutic interventions elicit reverse remodeling (RR), a highly variable myocardial response that ranges from none to total ventricular structural/functional recovery. However, HF patients present several comorbidities and medications that mask a comprehensive molecular knowledge of RR and hinder the identification of potential biomarkers of its progression or prognosis. Therefore, instead of using this heterogeneous population or even animal models to understand myocardial remodeling, we propose studying pregnancy-induced cardiovascular remodeling and postpartum-induced RR.
ObjectivesTo assess cardiovascular functional and structural adaptations during pregnancy and in postpartum, characterizing the associated molecular changes; as well as to explore the impact of hypertension, obesity and diabetes on these processes.
MethodsWe will perform echocardiography and assess endothelial function and arterial stiffness (EndoPAT® and pulse wave velocity, respectively) and assess potential markers of remodeling and RR using plasma and urine samples from pregnant women. To translate to a HF context, we will determine the impact of risk factors (hypertension, obesity and diabetes) by studying subgroups of pregnant women with these comorbidities.
ResultsNot applicable.
ConclusionWe are convinced that understanding the impact of these comorbidities in such a homogeneous population, such as pregnant women, provides a valuable model to unveil the most relevant pathologic and often masked signaling pathways underlying cardiac remodeling and incomplete RR in a heterogeneous population, such as HF patients. Moreover, we expect to identify potential novel biomarkers of RR progression/prognosis more easily.
A insuficiência cardíaca (IC) é uma das principais causas de morbimortalidade do mundo. Inúmeros contextos patológicos com início na sobrecarga crónica de volume/pressão do ventrículo esquerdo, e desenvolvimento de hipertrofia com posterior progressão para disfunção diastólica e sistólica, culminam num quadro de IC. Deste modo, as intervenções terapêuticas disponíveis visam promover o processo de remodelagem reversa (RR), cuja resposta miocárdica se tem demonstrado muito variável, desde a ausência à total recuperação ventricular. De facto, os doentes com IC apresentam diversas comorbilidades e terapêuticas farmacológicas associadas, que poderão dificultar caracterização molecular do padrão de RR presente, bem como a identificação de potenciais biomarcardores de progressão/prognóstico da mesma. Por tal facto, propomos o estudo da remodelagem cardiovascular e RR no contexto da gestação e pós-parto, uma vez que o modelo da mulher grávida surge de uma população mais homogénea do que a de com IC, superando, também, as limitações do uso do modelo animal.
ObjetivosAvaliar as adaptações cardiovasculares estruturais/funcionais durante a gravidez e o pós-parto, caracterizando molecularmente as mesmas; bem como explorar o impacto da hipertensão arterial (HTA), obesidade e diabetes mellitus (DM) nestes processos.
MétodosEste projeto inclui um conjunto de avaliações, nomeadamente ecocardiográfica, da função endotelial (EndoPAT®) e rigidez miocárdica(velocidade de onda de pulso); bem como a pesquisa de potenciais biomarcadores plasmáticos e de urina de RR. Adicionalmente, a amostra de estudo será analisada em subgrupos definidos de acordo com o fator de risco presente (HTA, obesidade e DM), de modo a determinar o impacto de cada um destes no processo de RR.
ResultadosNão aplicável.
ConclusãoAcreditamos que compreendendo o impacto de cada uma destas comorbilidades numa população homogénea, a mulher grávida, conseguiremos descobrir e explorar relevantes vias de sinalização patológicas presentes na remodelagem cardíaca e RR incompleta, característica em doentes com IC. Adicionalmente, pretendemos identificar novos biomarcadores de progressão/prognóstico da RR.
Pregnancy is a physiological condition in which hemodynamic overload is required to meet the increased demands of the growing fetus. These changes result from pregnancy-associated sympathetic and hormonal stimulation and consist of increased preload derived from blood volume expansion, and reduced afterload, derived from decreased peripheral vascular resistance.1–3 On echocardiogram, pregnancy adaptations result in non-pathological left ventricle (LV) eccentric hypertrophy, impairment of diastolic function with left atrium (LA) enlargement1,2,4 and reduced LV longitudinal and circumferential shortening with a compensatory increase of radial thickening so that myocardial performance maintains preserved ejection fraction.2,4 Recently, three-dimensional (3D) speckle tracking echocardiography has shown a reduction of myocardial deformation as an adaptive response to preload, afterload and LV geometry.4 Additionally, progressive blood volume increase leads to LA dilatation compromising contractility.1 As LA function critically modulates LV filling, reduced LA strain may justify the progressive, yet subtle, LV diastolic deterioration observed in pregnancy.1
Cardiovascular adaptations during pregnancy begin in the first trimester, peak in the second trimester and stabilize during the third trimester.3 However, the effect of these adaptations on LV diastolic function, LA and LV filling pressures are variable, and their determinants need to be clarified.1,2
Literature on post-partum is scarce and largely describes changes associated with peripartum cardiomyopathy and preeclampsia.5,6 Most studies state that in physiologic conditions, women's hearts fully recover and that global and segmental myocardial performance normalize to their pre-pregnancy structure and function in a process known as reverse remodeling. (RR).1,3,4 So far, no study has used pregnant women as a model to assess the impact of comorbidities on RR and its underlying mechanisms or resorted to plasma or urine samples of such a uniform population to identify potential signaling pathways or biomarkers of cardiac disease progression. The levels of brain natriuretic peptide (BNP) and high-sensitive troponin I (hs-TnI) have been described as increased immediately after delivery.7,8 On the one hand, increased levels of BNP are reported to have a protective role at post-partum in response to atrioventricular stretching and cardiac volume enlargement as well as to progressive hemoglobin decrease due to delivery bleeding.7,8 On the other hand, increased hs-TnI levels one week after delivery may be explained by transient myocyte injury with or without pathological cardiac remodeling even in pregnant women.8 Interestingly, BNP and hs-TnI were considered the best predictors of LA volume and LV mass indexes, respectively, in cardiac remodeling and RR processes.8
Hypertension, diabetes and obesityComorbidities, such as hypertension, obesity and diabetes, influence cardiac remodeling and RR during pregnancy/post-partum and impact the maternal future cardiovascular risk and mortality.6,9 Women who develop hypertensive disorders during pregnancy reveal post-partum subclinical cardiac dysfunction, reduced LV relaxation and left ventricular hypertrophy, following increased peripheral vascular resistance.10,11 This cardiac functional deterioration described as a consequence of hypertension is also associated with increased hs-TnI values, which reinforces the presence of a concomitant cardiac anatomical adaptation/remodeling.10 Even under optimal glucose management, gestational diabetes induces subtle diastolic dysfunction, impaired global longitudinal strain combined with increased LV mass compared to healthy pregnant women and, despite improving after delivery, these changes persist at least 6 months after delivery.12 Indeed, persistent diastolic dysfunction in post-partum raises the risk of future peripartum or another cardiomyopathy.10 Also, overweight and obese pregnant women reveal reduced myocardial performance and increased LV mass during gestation, specifically diastolic dysfunction at term.13 Systemically, marked endothelial dysfunction, arterial stiffness and increased risk of venous thromboembolism have been consistently described in the post-partum period.9,14
Lifestyle and maternal chronic stressMaternal lifestyle and chronic stress also influence cardiac remodeling/RR during the pregnancy and post-partum period. To prevent maternal complications and reduce the incidence of preeclampsia, gestational hypertension or gestational diabetes,15 the American College of Obstetricians and Gynecologists recommends practicing aerobic and resistance exercises with moderate intensity at least 20–30 min/day throughout a healthy pregnancy.16 Several factors assure aerobic capacity and circulatory reserve enough to support pregnant women and fetus needs during exercise, such as pregnancy-induced cardiovascular remodeling, enhanced stroke volume and increased minute ventilation by up to 50% caused by tidal volume rise.15,16
Adequate maternal nutrition in quantity and quality also reduces the likelihood of developing gestational DM and preeclampsia.17 A higher intake of red meat and processed food has been shown to increase the risk of developing pregnancy complications, such as gestational DM.18 Red meat consumption is also associated with higher plasma trimethylamine N-oxide (TMAO) levels, identified as an independent risk factor for cardiovascular disease and atherosclerosis. TMAO is generated from dietary phosphatidylcholine and l-carnitine metabolism by gut microbiota. Elevated TMAO levels negatively impact on the homeostasis of blood glucose and lipids, correlating with systemic inflammation and endothelial dysfunction.18,19 Given the relevant pro-inflammatory effect of TMAO, positive associations were described between both preeclampsia severity and gestational DM and plasma TMAO concentration.18,19
Malnutrition and chronic stress significantly impact maternal blood composition and homeostasis, changing placental cytokines and endocrine factors and altering placental gene expression at the maternal-fetal interface.20 Thus, the disruption of immune tolerance, progression of trophoblast invasion and spiral artery modification (interfering in the transport of nutrients), or de novo placental production of neuroactive molecules can lead to maternal or obstetric complications (such as preeclampsia) and impaired fetal development, such as hypoxia, ischemia, intrauterine growth restriction or immune dysregulation.20
Chronic stress response activates the hypothalamic–pituitary–adrenal axis leading to abnormally increased cortisol production, imposing a major acute and chronic impact on the cardiovascular system.21 A maternal stress-induced pro-inflammatory environment leads to impaired prostacyclin and nitric oxide production, resulting in endothelial dysfunction as assessed by EndoPAT.22
Stretch-induced complianceIn the cardiovascular system, homeostasis is maintained due to continuous cardiac adaptations to hemodynamic conditions. An increase in venous return (preload) triggers an acute myocardial stretch characterized by an immediate rise in contractility (the Frank-Starling mechanism), followed by a progressive increase known as the slow force response.23 Concomitantly to these adaptations, we have described a novel adaptive mechanism following acute myocardial stretch, named stretch-induced compliance (SIC), whereby myocardial compliance increases due to cGMP-PKG pathway activation and titin phosphorylation.23 Most importantly, we showed that this mechanism was impaired in pathological hypertrophy.23 In contrast, we expect enhanced SIC mechanism in in physiologic hypertrophy, as in pregnancy. Therefore, to explore SIC, we will perform a passive leg elevation maneuver by lifting the lower limbs from the horizontal plane up to 45° to predict the volume responsiveness and measure the preload reserve.
HypothesisSeveral pathologic conditions associated with volume or pressure overload impose maladaptive remodeling and trigger pathologic hypertrophy. Over time this response leads to diastolic and/or systolic dysfunction and HF. Most therapeutic interventions aim to relieve LV overload, induce regression of hypertrophy and recover LV function through RR. Indeed, the extent of RR in pathologic conditions is a surrogate parameter for patient prognosis: when RR is complete (cardiac recovery), it nearly restores survival to the pre-morbid state. However, there is a high variability of LV response, ranging from incomplete RR to complete RR. Since incomplete remodeling is associated with a poor prognosis, identifying novel strategies to reverse or predict this process is of utmost importance. Even considering the limitations of comparing pregnant women with HF patients (sex- and age-associated differences), we trust we can find valuable clues in pregnancy and post-partum remodeling; therefore, we hypothesize that:
- 1.
Pregnant women represent a valuable model to study the impact of comorbidities and lifestyle in the process of cardiac and vascular remodeling (during pregnancy) and RR (during post-partum), considering that they represent a more uniform population compared to HF patients (fewer medication and less confounding factors).
- 2.
Including subgroups of pregnant women with such comorbidities offers a platform to uncover potential biomarkers and important mechanisms underlying cardiac remodeling and RR that can be subsequently translated/validated in the context of HF.
- 3.
Lastly, pregnant women represent a tool to study the SIC mechanism, which will likely be exacerbated considering the cardiac adaptations to blood volume during pregnancy and post-partum.
Pregnancy induces cardiac remodeling that rapidly normalizes in the post-partum. Post-partum cardiac recovery may provide important new insights into the process and extent of RR. Therefore, the goal of this protocol is to assess cardiovascular functional and structural adaptations during pregnancy and meticulously characterize changes in the post-partum using innovative echocardiographic methods. Moreover, we aim to characterize these adaptations in healthy, hypertensive, diabetic and obese pregnant women at baseline and in response to an acute myocardial overload/stretch and to clarify the underlying mechanisms (SIC mechanism). To achieve these endpoints (Table 1), our specific goals are to:
- 1.
Characterize functionally and structurally cardiac remodeling (during pregnancy) and RR (at post-partum) and assess the impact of comorbidities (hypertension, diabetes and obesity) and lifestyle (physical exercise, nutrition and chronic stress) on these cardiac adaptations through innovative echocardiographic methods.
- 2.
Assess pregnancy and post-partum arterial stiffness and endothelial function, as well as the impact of comorbidities and lifestyle on these vascular adaptations.
- 3.
Determine potential biomarkers of cardiac remodeling (LV hypertrophy and/or functional recovery) by assessing plasma levels of markers of cardiac hypertrophy, extracellular matrix turnover, cyclic guanosine monophosphate (cGMP) and inflammatory and adhesion molecules in pregnant women by correlating these levels with LV systolic and diastolic parameters during pregnancy and post-partum.
- 4.
Differentiate the molecular signature of cardiac remodeling and RR between the four subgroups of pregnant women (healthy, hypertensive, diabetic, and obese).
- 5.
List urine protein profiles among four study groups and untangle potential associations with cardiovascular RR patterns after determining proteomic signatures by liquid chromatography with tandem mass spectrometry.
- 6.
Clarify the SIC mechanism at the peak of cardiac remodeling and during RR using echocardiography and assess the impact of comorbidities and lifestyle.
Primary and secondary endpoints.
| Primary endpoint |
| • LV mass (6–8 weeks, 6–7 months and 1 year) – RR |
| Secondary endpoints |
| • Echocardiographic characterization of cardiac remodeling and RR: |
| ∘ LA volume, LV end-systolic and end-diastolic volumes; |
| ∘ Septum and posterior wall thickness; |
| ∘ Global longitudinal and circumferential strain; |
| ∘ LA longitudinal strain; |
| ∘ 3D LV volumes and ejection fraction quantification; |
| ∘ Systolic function: ejection fraction (Simpson biplane); |
| ∘ Diastolic function: E and A waves, E/A ratio, E wave deceleration time, e′ medial, e′ lateral, E/e′ ratio, isovolumetric relaxation time; |
| ∘ Right atrium volume; |
| ∘ Right ventricle assessment: right ventricular index of myocardial performance, fractional area change, tricuspid annular plane systolic excursion, systolic pulmonary artery pressure, pulsed Doppler peak velocity at the annulus. |
| •Vascular stiffness assessment: |
| ∘ Pulse wave velocity |
| ∘ Augmentation index. |
| • Endothelial function evaluation: |
| ∘ Reactive hyperemia index |
| • Biomarkers of inflammation and endothelial cell activation: |
| ∘ E-selectin, |
| ∘ P-selectin, |
| ∘ Soluble intercellular |
| ∘ Vascular adhesion molecule (ICAM and VCAM). |
| • Biomarkers of cardiac injury: |
| ∘ BNP |
| ∘ NT-proBNP |
| ∘ TnI |
| • Biomarkers of cardiac repair: |
| ∘ ST2/IL33 receptor |
| • Biomarker of inflammation: |
| ∘ C-reactive protein |
| • Biomarkers of neurohormonal activity: |
| ∘ Aldosterone |
| ∘ Renin. |
| • Biomarkers of endothelial function: |
| ∘ Plasminogen-activator inhibitor type 1 |
| ∘ Homocysteine |
| ∘ Relaxin-2 |
| • Biomarkers of fibrosis and extracellular matrix turnover: |
| ∘ Tissue-inhibitor-of-matrix-metalloproteinases-1, |
| ∘ Procollagen I C terminal propeptide, |
| ∘ Lysyl-oxidase, |
| ∘ MMPs expression. |
| • Urinary proteomic profiles using liquid chromatography with tandem mass spectrometry. |
This is a prospective cohort study. Adult pregnant women (>18 years-old) will be recruited during their first medical appointment (in the 1st or 3rd trimester of pregnancy) at the Obstetrics Departments of Centro Hospitalar Universitário São João (CHUSJ), and Unidade Local de Saúde de Matosinhos-Hospital Pedro Hispano (ULSM-HPH) or may register via online forms available at https://perimyrobb.wordpress.com/. Exclusion criteria are pre-existing cardiomyopathy, renal disease, chronic obstructive airway disease, active systemic infection, genetic syndromes or type-1 DM.
SubgroupsThe study groups are as follows: (1) healthy pregnant women (without cardiovascular risk factors); (2) hypertensive pregnant women (if systolic blood pressure(SBP) ≥140 mmHg and/or diastolic blood pressure(DBP) ≥90 mmHg measured in office or in-hospital, which precedes pregnancy or developed before 20 weeks of gestation) or gestational hypertensive (if SBP ≥140 mmHg and/or DBP ≥90 mmHg measured in office or in-hospital, which develops after 20 weeks of gestation and usually resolves within 42 days post-partum); (3) gestational diabetic women (if 92≤fasting-glucose≥126 mg/dL at 1st trimester or ≥180 mg/dL or ≥153 mg/dL 1 or 2 hours after an oral glucose tolerance test (75 g oral glucose load) performed at 24-28 pregnancy weeks; (4) obese pregnant women (if body mass index ≥30 kg/m2 before pregnancy).
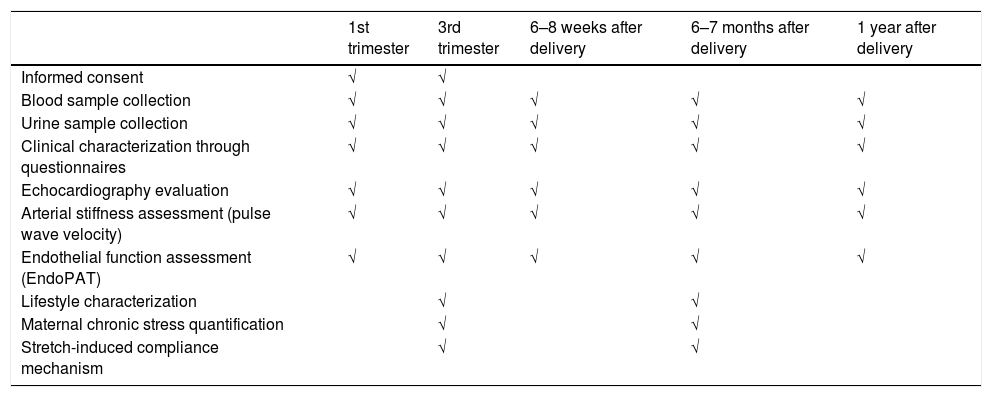
ProceduresA qualified and trained multidisciplinary clinical research team will conduct all study procedures. Participants will undergo echocardiographic evaluation, pulse wave velocity, EndoPat®, blood and urine sample collection at the Department of Surgery and Physiology of the Faculty of Medicine of the University of Porto, during the following time points (Table 2):
- 1.
First trimester of pregnancy – 1T (11–14 weeks), before cardiac remodeling (baseline conditions).
- 2.
Third trimester – 3T (30–35 weeks), at peak of cardiac remodeling when cardiovascular adaptations are expected to be most prominent.
- 3.
Post parturition (PP1, 6–8 weeks; PP2, 6–7 months; and PP3, 1 year after delivery) – during RR to assess cardiovascular recovery.
Planned follow-up for all participants.
| 1st trimester | 3rd trimester | 6–8 weeks after delivery | 6–7 months after delivery | 1 year after delivery | |
|---|---|---|---|---|---|
| Informed consent | √ | √ | |||
| Blood sample collection | √ | √ | √ | √ | √ |
| Urine sample collection | √ | √ | √ | √ | √ |
| Clinical characterization through questionnaires | √ | √ | √ | √ | √ |
| Echocardiography evaluation | √ | √ | √ | √ | √ |
| Arterial stiffness assessment (pulse wave velocity) | √ | √ | √ | √ | √ |
| Endothelial function assessment (EndoPAT) | √ | √ | √ | √ | √ |
| Lifestyle characterization | √ | √ | |||
| Maternal chronic stress quantification | √ | √ | |||
| Stretch-induced compliance mechanism | √ | √ |
Conventional transthoracic echocardiography evaluation with a 3 MHz phased-array probe (ACUSON SC2000 PRIME™) will be performed by a single operator and measurements will be obtained from standard views according to European Society of Cardiology recommendations for chamber quantification and diastolic function evaluation.24,25 At least three cardiac cycle images will be acquired for data analysis. Two certified cardiologists will independently analyze, interpret and harmonize the results. Appendix A shows the detailed protocol of transthoracic echocardiogram image acquisition and measurements.
Myocardial deformation will be assessed in LA and LV through strain and strain rate analysis by Syngo Velocity Vector Imaging software, version 3.5 (Siemens Healthcare, Erlangen, Germany). The images will be stored in digital imaging and communications in medicine format at 30 frames/second and analyzed offline posteriorly. The endocardium will be tracked manually using a point-and-click approach while the system automatically traces the epicardium and generates six segments. The tracing will be readjusted manually to increase tracking accuracy, and strain curves for each segment will be generated. Longitudinal strain will be calculated using apical two-, three-, and four-chamber views and averaged. Radial and circumferential strain will be assessed using the parasternal short axis view at the papillary muscle level. Left atrial strain and strain rate curves will be measured from standard non-foreshortened apical four-chamber view, whose border will be outlined manually. Regional peak strain and time to peak strain will be averaged to obtain global peak strain, strain rate, and time to peak strain. Time to peak strain and strain rate in systole and diastole will be recorded for each strain measurement and then adjusted for heart rate.
Three-dimensional data set swill be used for 3D software to quantify right ventricular and LV end-diastolic and end-systolic volumes, ejection fraction and mass.
Lastly, a specific echocardiographic protocol will be performed during 3T and PP2 visits to evaluate the response to an acute myocardial stretch. This protocol consists of performing a brief echocardiography exam in left lateral decubitus at baseline (T0), immediately after legs elevation to 45° (T1, passive leg raising) and after 15 minutes in this position (T2). This maneuver increases cardiac venous return, triggering myocardial stretch and the SIC mechanism.23 Blood samples will be collected in T0 and T2.
Vascular stiffness and endothelial function evaluationBrachial blood pressure will be measured in the non-dominant arm after 10 minutes of rest. Two non-invasive methods will be used to evaluate vascular remodeling and RR: EndoPAT™ 2000 system (Itamar Medical, Israel) will determine the endothelial function, based on non-invasive peripheral arterial tone (PAT) signal technology measuring endothelium-mediated changes in vascular tone using two finger probes in each index finger. Participants lay supine in bed or in a reclining chair unless the supine position impairs their breathing. After a five-minute equilibration period, the blood pressure cuff will be inflated on the experiment arm to supra-systolic pressure (at least 60 mmHg above the patient's SBP or between 200 and 300 mmHg) and held for a five-minute occlusion period. Then, the pressure will be released and reactive hyperemia will be measured for five minutes to assess the Reactive hyperemia index (RHI=(C/D)/(A/B)×basal correction).
The Complior® (Alam Medical, France) device will be used to quantify the arterial stiffness by carotid-femoral pulse wave velocity (cf-PWV), calculated from carotid-femoral distance/transit time, and peripheral augmentation index, defined as the ratio of late systolic pressure to early systolic pressure. In this context, the participant lays supine on the exam bed and relaxes to stabilize their heart rate and blood pressure. One sensor is placed at the carotid artery level and another at the femoral artery level until the software stabilizes. Each patient will undergo at least two cf-PWV measurements (whose difference between them should be ≤0.5 m/s) in each session. The Complior Analyse software displays the pulse wave velocity and the central pressure waveform analysis.
Determination of potential plasma biomarkersFreshly collected blood will be used to isolate endothelial cells, which will subsequently be analyzed on a FACS Canto II flow cytometer to assess the expression of E-selectin, P-selectin, soluble intercellular and vascular adhesion molecules. We will also assess plasma levels of circulating cGMP, TMAO and relaxin-2, markers of cardiac injury, markers of cardiac repair, inflammatory markers, markers of neurohormonal activity, markers of endothelial function and markers of fibrosis and extracellular matrix turnover (Table 1) using ELISA kits following manufacturer instructions.
Urinary proteomic profileFifty milliliters of first morning urine samples will be collected, centrifuged (2370 g, 15 minutes, 4°C), and stored at −80°C at each visit (1T, 3T, PP1, PP2 and PP3). Urine protein will be concentrated using centrifugal concentrators (cutoff-10kDa) and quantified by a standard bicinchoninic acid assay.
Proteome will be analyzed following a label-free shotgun approach to disclose the urinary protein profile changes induced by each cardiovascular risk factor. Briefly, for each sample, 100 μg of protein will be reduced by incubation with dithiothreitol and alkylated in the dark. The sample will be diluted with ammonium bicarbonate and the proteins trypsin-digested overnight (16 h, 37°C). After sample cleanup (C18-resins), peptides will be analyzed using high-performance liquid chromatography (LC) coupled to a high-resolution mass spectrometer operating in tandem (MS/MS).
Raw data will be processed, and quantitative label-free analysis of the LC-MS/MS data will be performed with MaxQuant or similar algorithms. The data will be searched against a database containing all proteins corresponding to humans in the Swissprot database, plus a list of common contaminants and all the corresponding decoy entries. No more than three missed cleavages will be allowed for trypsin. Peptides and proteins will be filtered out using a 1% false discovery rate.
Lifestyle characterization and maternal chronic stress quantificationThe lifestyle characterization will be based on nutritional, physical activity and chronic stress assessment through the application of validated Portuguese questionnaires, during 3T and PP2, except the Pregnancy Physical Activity Questionnaire, which will be applied only during pregnancy:
- 1.
As the Mediterranean diet is recognized as the healthier dietary pattern associated with a reduction of cardiovascular disease incidence in high risk individuals, a Mediterranean Diet Adherence Screener (MEDAS) Questionnaire composed of 14 items related to food intake habits and frequency of consumption of foods will be applied.26 Each positive answer equals one point and a MEDAS final score >9 indicates adherence to this diet.
- 2.
The Pregnancy Physical Activity Questionnaire27 is composed of 36 questions that quantify the duration, frequency and intensity of 32 physical activities (from household/caregiving, occupational activities, sport/exercise to running or inactivity). These categories are subdivided into different intensity levels quantified by metabolic equivalents (METs), namely sedentary (<1.5 METs), light (1.5–3.0 METs), moderate (3.1–6.0 METs) or vigorous (>6.0 METs).
- 3.
The Perceived Stress Scale is composed of 14 items to assess the perception of general psychological chronic stress.28 Each of the 14 items is assessed using a five-point Likert scale. In addition, a Portuguese version of the Prenatal Distress Questionnaire will be used to assess specific pregnancy stress.29 This questionnaire focuses on specific concerns of pregnant women ranging from medical problems, physical symptoms, bodily changes, childbirth to baby health, and contains 12 questions, classified using a five-point Likert scale.
To quantify chronic stress further, a band of approximately 80 hairs will be collected from pregnant women in the third trimester of pregnancy and six to seven months after delivery to assess chronic cortisol levels. Hair cortisol has proved to be a valid and reliable method to assess long-term cortisol secretion, showing some advantages to cortisol quantification from biofluids (such as saliva, blood and urine sample), which reflect acute cortisol changes and are influenced by circadian fluctuations.30
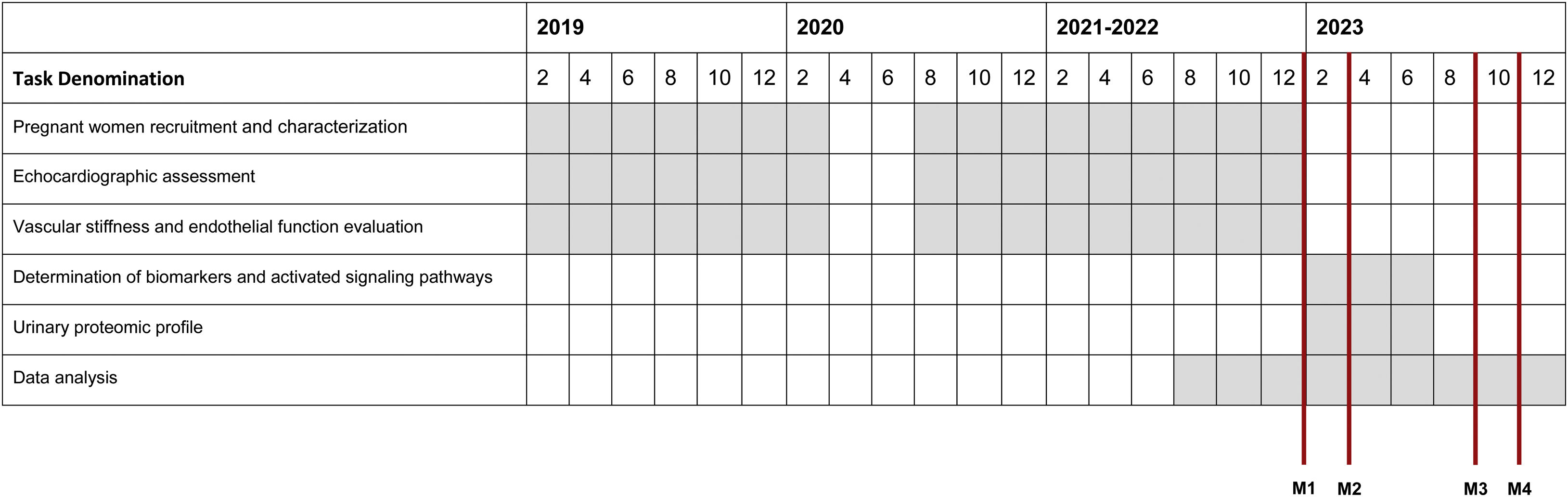
Ethical considerationsAll eligible participants should be able and willing to provide written informed consent to be included in this study. The Ethics Committees of CHUSJ and ULSM-HPH approved the study (ID 201/18 and ID 154/20/RS, respectively). The confidentiality and data anonymity comply with the Declaration of Helsinki of 1964, revised in Fortaleza, in 2013. Participant recruitment began in February 2019.
Data collection and storageThe clinical characterization of the study sample will include maternal cardiovascular health, obstetric and perinatal outcomes, maternal health-related habits, maternal smoking habits, drinking and medical history. In addition, obstetric (gestational hypertension, gestational diabetes, type of delivery, gestational age at delivery) and perinatal (fetal death, birth weight, Apgar score) outcomes will also be registered. These data will be collected through the application of questionnaires and collected from medical records whenever necessary.
Clinical parameters, echocardiographic and plasma determinations, reactive hyperemia index, augmentation index and pulse wave velocity values will be gathered on a database in IBM-SPSS-Statistics, version 25.0 and subsequently analyzed using R programming language (Table 3).
Schedule.
Milestones
M1: Complete study sample recruitment.
M2: Detailed characterization of cardiovascular remodeling and reverse remodeling in pregnant women, by echocardiographic (primary and secondary endpoints), pulse wave velocity and EndoPAT® analysis (secondary endpoint).
M3: Determination of potential plasma biomarkers – secondary endpoint.
M4: Determination of urinary proteomic profiles – secondary endpoint.
All data management will comply with professional confidentiality and use pseudonymization. Only PERIMYR team members will have access to the data. An exclusively dedicated server will be used to store study information and its access will be restricted to the study team. Data collection and management comply with General Data Protection Regulation (GDPR) 2016/679 27 April 2016 and 58/2019 Law (Portugal). Biological samples will be kept throughout the project and destroyed five years after study completion.
Statistical analysisFor sample size estimation, we considered the pre-specified analysis of LV mass index regression at six to eight weeks after delivery. Thus, a sample size of 25 patients per group was estimated to allow the detection of a difference in means of 9.8 g/m2 (power of 90% and α=0.05), assuming a standard deviation of 12.3 g/m2 in each group and a conservative third trimester/seven post-partum weeks measurement correlation of 0.2 to account for the intrasubject biological variation. Considering a potential drop-out rate of 20%, the final estimated sample size needed in each allocation group is 30 participants.
Continuous variables will be expressed by means and standard deviation or by median, minimum and maximum as adequate. Data normality will be checked after examining histograms and Q-Q plots. Absolute values and relative frequencies will be presented for categorical variables.
One-way analysis of variance or Kruskal-Wallis test will be performed to evaluate differences among study groups for continuous variables and chi-square analysis will be used for categorical variables. Linear mixed-effects models will be used to explore the longitudinal nature of the data. The analysis of cardiac mass regression during reverse remodeling will be performed by repeated-measures analysis of covariance adjusted for clinically relevant variables.
Linear mixed-effects models will be used to explore the longitudinal nature of the data. The analysis of cardiac mass regression during reverse remodelling will be performed by repeated-measures analysis of covariance (ANCOVA) adjusted for clinically relevant variables.
Principal component analysis (PCA) and partial least squares - discriminant analysis (PLS-DA) will be used to explore the multivariate nature of the data, the potential associations between the qualitative and quantitative analyses, as well as, with echocardiographic, plasma and vascular parameters. PCA (unsupervised technique) and PLS-DA (supervised technique) will be performed to uncover clusters within the samples of the participants and to assess relationships among the diverse sets of clinical and molecular variables. Both approaches aim to reduce the high-dimensional nature of the dataset, simplifying the analysis, improving the reasoning about the potential clustering of samples and the putative variables (clinical and molecular) network topology. This approach will improve our understanding of the impact of each comorbidity on the molecular events related with cardiac RR. In this scenario, PCA will be used for exploratory purposes and data compression will be fed into linear and logistic multivariable models, whereas the application of PLS-DA will be used as a hypothesis generator. p<0.05 will be deemed significant.
Missing dataMissing data are one potential limitation if participants fail to attend the cardiovascular appointment without the possibility of rescheduling, as was the case during the COVID-19 pandemic (Appendix B). Maternal lifestyle and clinical characterization may alternatively be registered by phone call or e-mail. However, quantitative variables of pulse wave velocity, EndoPAT™ and echocardiographic assessment cannot be measured without the participant's presence. In this case, we will focus the data analysis exclusively on the third trimester and six to seven months and/or using imputation methods.
ConclusionPERIMYR represents the first study specially designed to use a population of pregnant women as a model to study cardiac remodeling, RR, potential biomarkers and the impact of comorbidities and lifestyle on RR, and to characterize the response to an acute myocardial stretch. This will occur by evaluating pregnant women at first and third trimesters and in three-time points during post-partum (at 6–8 weeks, 6–7 months and one year). This idea stems from the fact that pregnancy represents an excellent and unique human model to investigate cardiovascular remodeling and RR under physiological (pregnancy-induced remodeling and post-partum associated RR) and pathological conditions, i.e., when pregnant women present hypertension, diabetes or obesity. Thus, PERIMYR can provide invaluable knowledge translatable to the context of HF. Although RR analysis is restricted to a pregnant women's cohort, including only female participants younger than HF patients, the use of pregnant women as a model offers several advantages. These include the reduction of several confounding risk factors present in the pathologic context of HF and outperforming the information derived from animal models.
FundingThis work was supported by João Porto Study Grant from the Portuguese Society of Cardiology, by RTP Maratona da Saúde 2017 and by national funds through FCT - Portuguese Foundation for Science and Technology, under the scope of the Cardiovascular R&D Center - UnIC (UIDB/00051/2020 and UIDP/00051/2020). A.F. Ferreira and M.J. Azevedo are supported by Foundation for Science and Technology (SFRH/BD/138925/2018 and SFRH/BD/144982/2019, respectively).
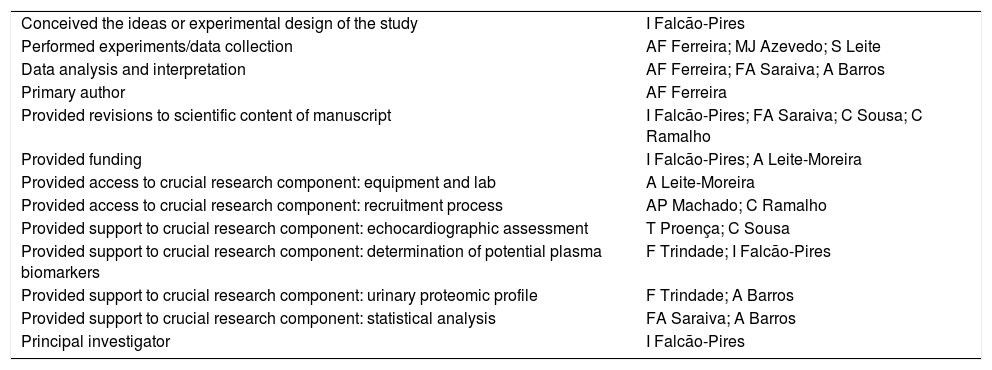
Authors’ contributions| Conceived the ideas or experimental design of the study | I Falcão-Pires |
| Performed experiments/data collection | AF Ferreira; MJ Azevedo; S Leite |
| Data analysis and interpretation | AF Ferreira; FA Saraiva; A Barros |
| Primary author | AF Ferreira |
| Provided revisions to scientific content of manuscript | I Falcão-Pires; FA Saraiva; C Sousa; C Ramalho |
| Provided funding | I Falcão-Pires; A Leite-Moreira |
| Provided access to crucial research component: equipment and lab | A Leite-Moreira |
| Provided access to crucial research component: recruitment process | AP Machado; C Ramalho |
| Provided support to crucial research component: echocardiographic assessment | T Proença; C Sousa |
| Provided support to crucial research component: determination of potential plasma biomarkers | F Trindade; I Falcão-Pires |
| Provided support to crucial research component: urinary proteomic profile | F Trindade; A Barros |
| Provided support to crucial research component: statistical analysis | FA Saraiva; A Barros |
| Principal investigator | I Falcão-Pires |
The authors have no conflicts of interest to declare.
Patient positioning:
1. Left lateral position (PLAX; PSAX; Apical A4C, A5C, A2C, A3C)
2. Supine position (suprasternal, subcostal)
General recommendations:
1. Attention to the quality of the ECG;
2. Perform measurements in at least 3 cycles;
3. Attention to the quality and framing of the Images – adjust gains, depth, focus, scale and baseline;
4. Doppler:
The Nyquist speed between 50 and 70 cm/s, (ideally, 64 cm/s);
B. Sample volume size: 5 mm;
5. Frame rate: 50–80 IPS;
6. Sweep speed: 100 mm/s;
7. Tissue Doppler scale: 20 cm/s.
| Checklist | |
|---|---|
| Acquisitions | Recommendations |
| Parasternal long axis | |
| 2D | Measurements: IVS, PWT, LVEDD, LVESD, LAD, sinuses of valsalva diameter, sinotubular junction diameter |
| 2D – focused on ascending Ao | Measure the diameter of the ascending Ao |
| Color Doppler focused on aortic valve | Evaluate coaptation and flow (type, direction) |
| Color Doppler focused on mitral valve | Evaluate coaptation and flow (type, direction) |
| M mode on aortic valve | Evaluate coaptation |
| M mode on mitral valve | Evaluate coaptation |
| 2D – focused on aortic valve | Measurements: aortic root |
| Parasternal short axis | |
| 2D – short axis of large vessels | Evaluate aortic valve morphology |
| Color Doppler in the aortic valve | If aortic regurgitation: evaluate mechanism |
| Color Doppler in the tricuspid valve | Evaluate flow (type, direction) |
| CW tricuspid valve | If tricuspid regurgitation: measure gradients |
| Color Doppler in the pulmonary valve | Evaluate flow (type, direction) |
| PW pulmonary valve | Quantification of pulmonary acceleration time and ejection time |
| 2D – mitral valve | Evaluate mitral valve morphology |
| Color Doppler mitral valve | Evaluate the coaptation of leafletsIf mitral regurgitation: evaluate mechanism |
| 2D – papillary muscles | Assess function and contractilityEvaluate the segment (s) involved |
| 2D – LV apex | Assess function and contractilityEvaluate the segment (s) involved |
| Apical 4 chambers view | |
| 2D | Assess function and contractility, calculate EF (Simpson method)Measure: LVEDV, LVESV, LA and RA volumes |
| LV apex plane | If poor function: assess the presence of thrombi |
| Color Doppler mitral valve | Evaluate coaptation and flow (type, direction) |
| PW mitral valve | Measure E and A waves, E/A ratio, deceleration time, isovolumic relaxation time |
| CW mitral valve | If regurgitation: calculate VTIIf stenosis: calculate THP and measure gradients |
| Color Doppler tricuspid valve | Evaluate flow (type, direction) |
| CW tricuspid valve | If tricuspid regurgitation: measure gradients |
| Tissue Doppler (PW) in the lateral mitral annulus | Measure lateral E′ peak velocity |
| Tissue Doppler (PW) in the medial mitral annulus | Measure medial E′ peak velocity |
| Tissue Doppler (PW) in the tricuspid ring | Measure E′, A′ and S′ peak velocity; |
| M mode on the lateral tricuspid ring | TAPSE |
| 2D focused on right ventricle | Measure: end-diastolic area and end-systolic area for FAC calculation; right ventricular basal and mid-cavity diameters and longitudinal diameterPW Tricuspid valve: measure E and A waves, E/A ratio, deceleration time, isovolumic relaxation time |
| Apical 5 chambers view | |
| Doppler (color) LVOT | Evaluate coaptation and flow (type, direction) |
| CW AoV | Measure gradients |
| PW in LVOT | If stenosis: calculate VTI |
| Apical 2 chambers view | |
| 2D | Assess function and contractility, calculate EF (biplane Simpson method)Measure: LVEDV, LVESV, LA volume |
| Doppler (color) mitral valve | Evaluate coaptation and flow (type, direction) |
| Apical 3 chambers view | |
| 2D | Assess function and contractility |
| Doppler (color) in LVOT and Ao | Evaluate coaptation and flow (type, direction) |
| Doppler (color) in mitral valve | |
| Suprasternal | |
| 2D | Evaluate ascending Ao morphology (calculate aortic arch and descending aorta diameter) |
| Color Doppler in the Ao and Ao descending arc | Evaluate flow (type, direction)Measure velocity |
| Pulsed Doppler in descending Ao | |
| Subcostal | |
| 2D of right ventricular wall | Measure end-diastolic right ventricular wall thickness |
| Doppler (color) IA septum | Measurements: DVAssess the presence of shunts/PFO |
| IVC, inferior vena cava | Measure diameterAssess respiratory variability (<50%/>50%) |
EF: ejection fraction; FAC: fractional area change; IVSd: septal interventricular septum in diastole; LA: left atrium; LVEDd: left ventricular internal diameter in diastole; LVEDV: left ventricular end-diastolic volume; LVESd: left ventricular internal diameter in systole; LVESV: left ventricular end-systolic volume; PFO: patent foramen ovale; PWd: posterior wall thickness in diastole; RA: right atrium; VTI: velocity time integral.
LV mass will be estimated using the following equation 0.8×(1.04×[(LVEDd+PWd+IVSd)3−(LVEDd)3]+0.6). In addition, the relative wall thickness will be calculated using the equation: 2×PWd/LVEDd. The biplane Simpson method will be used to estimate LV end-diastolic and end-systolic volumes, stroke volume and ejection fraction (calculated through [LVIDd]3−[LVIDs]3/[LVIDd]3×100%). LV cardiac output (CO) will be calculated by LV stroke volume×Heart rate. Systemic vascular resistance will be determined using the equation: 80×(mean arterial pressure/LV CO) (dyn.s.cm−5) and mean arterial pressure will be calculated by the formula: SBP+((2×DBP)/3). All cardiac volumes and LV mass will be indexed to the body surface area of the participants (0.007184×height (cm)0.725×weight (kg)0.425). The right ventricular index of myocardial performance will also be calculated by the isovolumic time ratio divided by ejection time.
The COVID-19 pandemic interfered in the progression of some PERIMYR tasks, including the recruitment process and the cardiovascular evaluation scheduling. Following the Portuguese Government recommendations for the pandemic period, an addendum was added to the protocol of PERIMYR to cope with the updated national recommendations from the national health system, such as: (1) transferring the clinical evaluation room to a research building outside the hospital environment and thereby minimizing the risk of exposure of the participants to COVID-19 infection; (2) disinfecting the room and all equipment and devices between participants; (3) use of personal protective equipment by the PERIMYR team and participants; (4) regular COVID-19 testing of the PERIMYR team members, and (5) suppressing the leg elevation maneuver to assess SIC mechanism to shorten the duration of each evaluation. The reformulated PERIMYR protocol was approved by Ethics Committee of Centro Hospitalar Universitário São João and University of Porto Task Force in May 2020.