Rotational atherectomy (RA) is widely used for the management of calcified coronary stenoses. However, there is limited data on its use, trends, and outcomes. We sought to report our twelve-year experience with RA and explore the trends and outcomes of percutaneous coronary intervention (PCI) with this device.
MethodsOur institutional PCI database was queried to identify all cases of RA-PCI performed between January 2009 and December 2020. We analysed peri-procedural outcomes and major adverse cardiovascular events (MACE) during follow-up: cardiovascular death, myocardial infarction, and target lesion revascularization.
ResultsFour hundred ten procedures (2.8% of total PCI volume) in 388 patients were included. Mean age was 72.3±9.3 years, 74.0% were male, 53.6% had diabetes, and 33.8% presented with acute coronary syndrome. There was a significant increase in median SYNTAX score (ptrend=0.003) and the proportion of type B2/C lesions (ptrend=0.003). Transradial access was preferred (60.0% overall) with a growing trend over time (ptrend=0.003). Maximum burr size was <1.75 mm in 88.0% of cases (burr-to-artery ratio of 0.49±0.07). Angiographic success rate was consistently high (96.6% overall). Complications were recorded in 9.0% of procedures, with a temporal decline (ptrend=0.029). Clinical follow-up was available for 357 patients (median time of 40 months). At one year, MACE rate was 12.1% with no significant temporal changes.
ConclusionsRA-PCI was a safe and effective procedure with a high rate of angiographic success and few complications, particularly in recent years, in line with significant technical improvements. The MACE incidence is acceptable considering the clinical risk and angiographic complexity.
A aterectomia rotacional (AR) é amplamente utilizada para facilitar o tratamento de lesões coronárias calcificadas. Contudo, os dados sobre a sua utilização e resultados são limitados. O nosso objetivo foi caracterizar a nossa experiência de 12 anos com a AR e explorar as tendências temporais e os resultados da intervenção coronária percutânea (ICP) com este dispositivo.
MétodosAnalisámos retrospetivamente os doentes submetidos a AR entre janeiro de 2009 e dezembro de 2020. Avaliámos os resultados imediatos do procedimento e a ocorrência de eventos cardiovasculares major (ECM) durante o follow-up: morte cardiovascular, enfarte agudo do miocárdio, reintervenção na lesão alvo.
ResultadosForam incluídos 410 procedimentos (2,8% do total de ICP) em 388 doentes. A média de idades foi 72,3±9,3anos, 74,0% eram do sexo masculino, 53,6% tinham diabetes. Houve um aumento significativo no score SYNTAX e no número de lesões B2/C (ptrend = 0,003). O acesso radial foi o preferido (60,0%) com tendência crescente ao longo do tempo (ptrend = 0,003). O tamanho máximo da oliva foi <1,75mm em 88,0% dos casos (relação oliva-artéria: 0,49±0,07). A taxa de sucesso angiográfico foi consistentemente alta (96,6%), enquanto as complicações decresceram (9%; ptrend = 0,029). A um ano, 12,1% dos doentes tiveram ECM, sem alterações temporais (follow-up médio de 40 meses para 357 pacientes).
ConclusõesA AR é uma técnica segura e eficaz, com elevada taxa de sucesso angiográfico e uma taxa decrescente de complicações em consonância com melhorias técnicas significativas. A incidência de ECM é aceitável tendo em conta a complexidade clínica e angiográfica desta população.
Severe coronary artery calcification is a marker of lesion complexity and an established risk factor for suboptimal angiographic results, peri-procedural complications, and adverse long-term outcomes in patients undergoing percutaneous coronary intervention (PCI). With increasing life expectancy and prevalence of diabetes, hypertension, and chronic kidney disease, the frequency of severely calcified lesions is growing.1–3 Calcification may preclude PCI success by hampering device delivery,4 damaging drug-eluting stents’ (DES) polymeric coating,5 disrupting drug delivery and elution kinetics,6 and preventing adequate stent expansion and apposition – predictors of stent thrombosis and restenosis.7–10 Furthermore, in the presence of eccentric calcification, balloon dilation may be biased towards more compliant noncalcified segments, risking vessel dissection11 or perforation.12
Lesion preparation is crucial to overcome these challenges and ensure adequate balloon dilation before stenting.13 Rotational atherectomy (RA) is widely used for this purpose. Originally, RA was intended for mechanical removal of the atherosclerotic plaque before or instead of balloon angioplasty, but initial interest was hampered by high target vessel failure rates.14 The advent of DES and consequent improvement in restenosis rates has led to a resurgence of interest in RA, now aiming to modify fibrocalcific plaques to facilitate optimal DES implantation.15
While it is clear that RA enables device delivery and procedural success in most calcified lesions, definitive evidence of long-term benefit is lacking.16,17 Furthermore, this approach has some drawbacks, including extended fluoroscopy time, longer learning curve, and higher rates of procedural complications (e.g., slow flow/no-reflow and coronary perforation) compared to balloon-based strategies.16–18 Therefore, despite decades of use, significant variability in patient selection and technical approach persists and its temporal changes have not been well described.
ObjectivesWe aimed to report our centre's experience with RA over the past twelve years and assess the temporal trends and outcomes of RA-PCI.
MethodsStudy design and populationWe conducted a retrospective cohort analysis of all RA procedures performed at our institution (Centro Hospitalar e Universitário de Coimbra, Portugal) between January 2009 and December 2020. Three hundred eighty-eight patients underwent RA-PCI for at least one lesion, in a total of 410 procedures. All were considered for this analysis, without inclusion/exclusion criteria. The study protocol was written according to the Declaration of Helsinki and approved by the institutional Ethics Committee (ID: CHUC-091-020; 27/08/2020). All patients provided written informed consent regarding the procedure, anonymized data collection, and analysis for research purposes.
Data collection and follow-upData were collected from our institutional PCI database, where information on all coronary interventions is prospectively recorded. Baseline characteristics were recorded as inserted by the interventional team and included patients’ demographics, relevant past medical history, cardiovascular risk factors, clinical presentation, and angiographic findings on the topography and severity of coronary artery disease (CAD). Technical details on arterial access, rotablation, balloon pre- and post-dilatation, stenting, use of other calcium modifying tools, intravascular imaging, temporary pacing, and hemodynamic support were documented. We performed an exploratory clinical follow-up analysis using our institution's electronic records and telephone interviews when patients were followed elsewhere.
Outcomes and definitionsAngiographic success was defined as the presence of less than 30% residual stenosis and grade 3 TIMI (thrombolysis in myocardial infarction) flow in all attempted lesions. Coronary perforation was defined as any dissection or intimal tear that extended through the full thickness of the arterial wall. Slow flow/no-reflow was defined as delayed or absent antegrade flow in the absence of an obstructive lesion. Burr entrapment was defined as any burr that got stuck within or beyond the lesion and could not be retrieved with standard operational methods.19 Two interventional cardiologists retrospectively assessed angiographic outcomes. Emergency coronary artery bypass grafting was defined as any emergency cardiothoracic surgical procedure to solve a PCI complication or after PCI failure. Peri-procedural myocardial infarction (MI) was recorded as registered by the medical team. All recorded events complied with the Fourth Universal Definition of type 4a MI.20 Follow-up data focused on major adverse cardiovascular events (MACE; defined as cardiovascular death, MI, and target lesion revascularization), all-cause mortality, target vessel revascularization and stent thrombosis, defined according to the Academic Research Consortium-2 criteria.21
Procedural detailsThe procedures were executed at a tertiary-care referral centre by 14 operators. All patients were pre-treated with aspirin and a loading dose of a P2Y12 receptor inhibitor. Intraprocedural anticoagulation was achieved with heparin (target activated clotting time of >250 or 200–250 secs when a glycoprotein IIb/IIIa inhibitor was administered). Access site and sheath size were determined by the anticipated maximum burr size and operator preference.
RA was performed with either the Rotablator™ or the Rotapro™ Rotational Atherectomy Systems (Boston Scientific Corporation, Natick, MA, USA). A continuous infusion of verapamil (5 mg), isosorbide dinitrate (10 mg), and unfractionated heparin (2500 IU) was administered through the device. A conventional 0.014-inch guidewire was used to facilitate navigation through the often-complex anatomy, and posteriorly exchanged for one of the dedicated RotaWire™ guidewires (Boston Scientific) via a microcatheter. As previously described,22 RA was carried out using a pecking motion of the burr, with short runs (15–20 secs) at a relatively low ablation speed (140000–150000 rpm). A reduction of more than 5000 rpm warranted immediate interruption of the ablation run to avoid burr lodging. Despite the preferential use of DES, some patients were exclusively treated with bare-metal stents or drug-coated balloons. Bioresorbable scaffolds were not implanted.
Statistical analysisCategorical variables were presented as frequency (percentage), while continuous variables were described as mean±standard deviation or median (interquartile range), according to the normality of the distribution assessed by the Kolmogorov–Smirnov test. Angiographic success and peri-procedural complications were analysed on a per-procedure basis, while follow-up data was analysed on a per-patient basis. Time trends in baseline characteristics and procedural outcomes were examined using the Cochran–Armitage test or the Kendall rank correlation coefficient. One-year event rates were based on Kaplan–Meier estimates. Calendar quartiles were compared using the log-rank test. Multivariate analyses were performed using logistic and Cox regression for procedural and clinical endpoints, respectively. In those models, we included statistically significant variables on univariate analysis and/or known predictors based on previous data. Effect sizes were reported using odds ratio (OR) or hazard ratio (HR) at 95% confidence interval (CI). Statistical analysis was performed with IBM SPSS Statistics, version 26.0 (IBM Corp., Armonk, NY, USA) and R 4.0.3 statistical software (R Core Team, Vienna, Austria), with an alpha significance level set at 0.05.
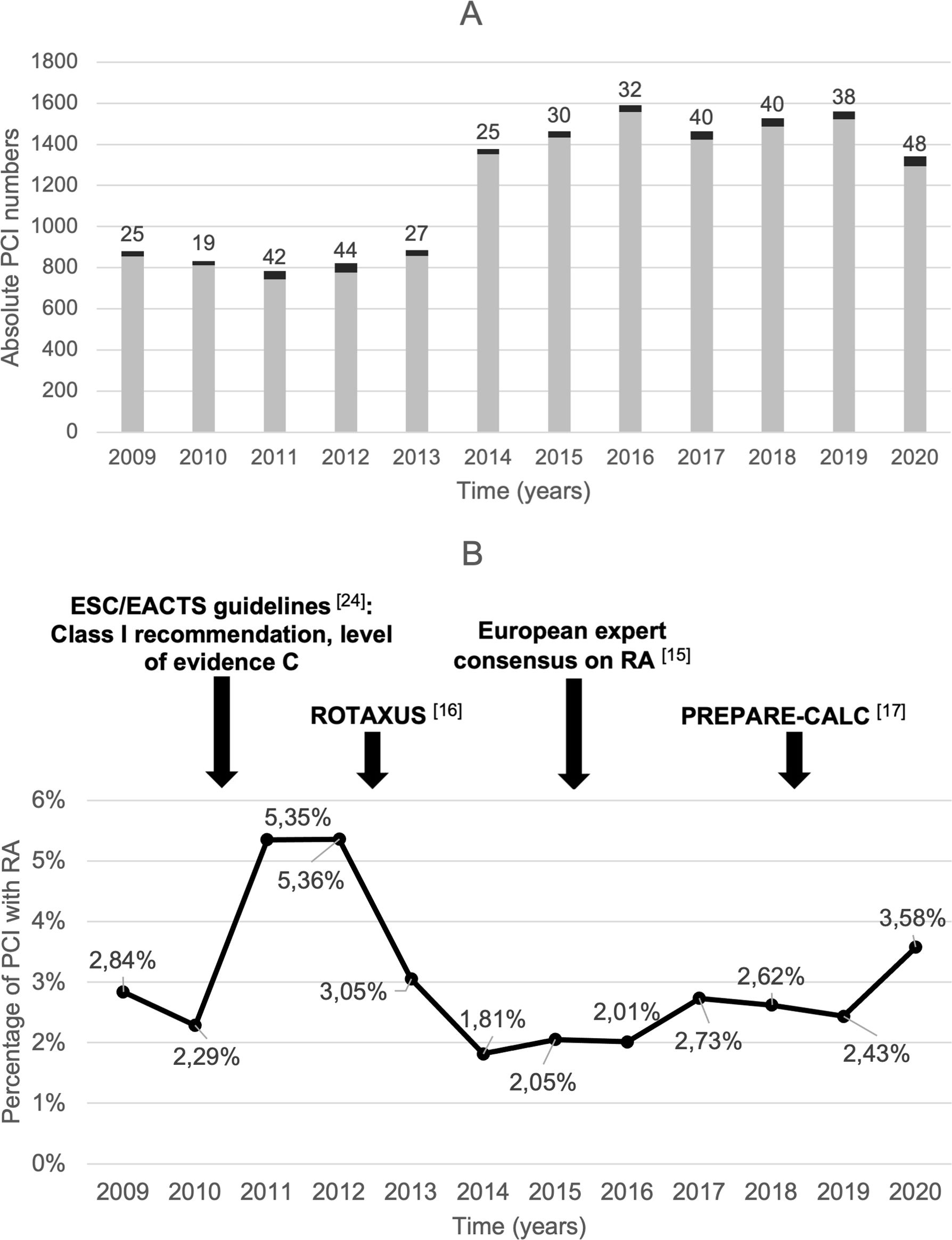
ResultsOf the total of 14527 PCIs performed during the study period, 410 (2.8%) included RA for at least one lesion, in a total of 388 patients. Figure 1 shows the variation in RA use over time.
Time trends in RA-PCI over the study period (2009–2020). (A) Absolute number of PCI with (black bars) and without (grey bars) RA; (B) percentage of total PCI that included RA with some of the most relevant clinical trials and guideline updates highlighted above. EACTS: European Association for Cardio-Thoracic Surgery; ESC: European Society of Cardiology; PCI: percutaneous coronary intervention; PREPARE-CALC: Comparison of Strategies to Prepare Severely Calcified Coronary Lesions; RA: rotational atherectomy; ROTAXUS: Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease.
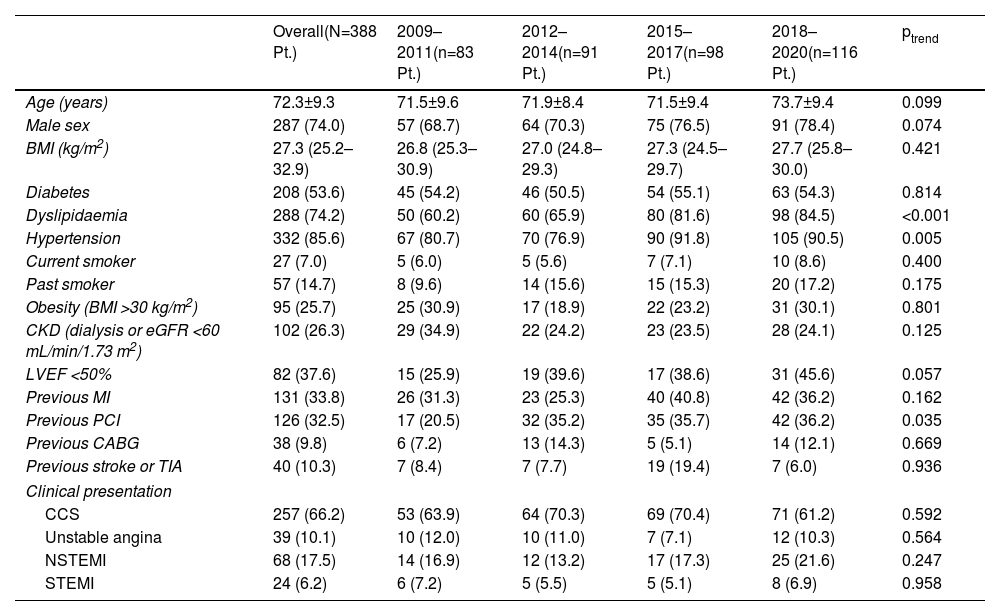
Baseline clinical characteristics are depicted in Table 1. Mean age was 72.3±9.3 years; patients were more often male (74.0%), and had high proportions of hypertension (85.6%), diabetes (53.6%), chronic kidney disease (26.3%), prior MI (33.8%), and impaired ejection fraction (37.6%). Initial presentation was an acute coronary syndrome in 33.8% of patients. The prevalence of dyslipidaemia (ptrend <0.001), hypertension (ptrend=0.008), and prior PCI (ptrend=0.035) increased significantly over the study period.
Temporal trends in baseline clinical characteristics by three-year intervals.
| Overall(N=388 Pt.) | 2009–2011(n=83 Pt.) | 2012–2014(n=91 Pt.) | 2015–2017(n=98 Pt.) | 2018–2020(n=116 Pt.) | ptrend | |
|---|---|---|---|---|---|---|
| Age (years) | 72.3±9.3 | 71.5±9.6 | 71.9±8.4 | 71.5±9.4 | 73.7±9.4 | 0.099 |
| Male sex | 287 (74.0) | 57 (68.7) | 64 (70.3) | 75 (76.5) | 91 (78.4) | 0.074 |
| BMI (kg/m2) | 27.3 (25.2–32.9) | 26.8 (25.3–30.9) | 27.0 (24.8–29.3) | 27.3 (24.5–29.7) | 27.7 (25.8–30.0) | 0.421 |
| Diabetes | 208 (53.6) | 45 (54.2) | 46 (50.5) | 54 (55.1) | 63 (54.3) | 0.814 |
| Dyslipidaemia | 288 (74.2) | 50 (60.2) | 60 (65.9) | 80 (81.6) | 98 (84.5) | <0.001 |
| Hypertension | 332 (85.6) | 67 (80.7) | 70 (76.9) | 90 (91.8) | 105 (90.5) | 0.005 |
| Current smoker | 27 (7.0) | 5 (6.0) | 5 (5.6) | 7 (7.1) | 10 (8.6) | 0.400 |
| Past smoker | 57 (14.7) | 8 (9.6) | 14 (15.6) | 15 (15.3) | 20 (17.2) | 0.175 |
| Obesity (BMI >30 kg/m2) | 95 (25.7) | 25 (30.9) | 17 (18.9) | 22 (23.2) | 31 (30.1) | 0.801 |
| CKD (dialysis or eGFR <60 mL/min/1.73 m2) | 102 (26.3) | 29 (34.9) | 22 (24.2) | 23 (23.5) | 28 (24.1) | 0.125 |
| LVEF <50% | 82 (37.6) | 15 (25.9) | 19 (39.6) | 17 (38.6) | 31 (45.6) | 0.057 |
| Previous MI | 131 (33.8) | 26 (31.3) | 23 (25.3) | 40 (40.8) | 42 (36.2) | 0.162 |
| Previous PCI | 126 (32.5) | 17 (20.5) | 32 (35.2) | 35 (35.7) | 42 (36.2) | 0.035 |
| Previous CABG | 38 (9.8) | 6 (7.2) | 13 (14.3) | 5 (5.1) | 14 (12.1) | 0.669 |
| Previous stroke or TIA | 40 (10.3) | 7 (8.4) | 7 (7.7) | 19 (19.4) | 7 (6.0) | 0.936 |
| Clinical presentation | ||||||
| CCS | 257 (66.2) | 53 (63.9) | 64 (70.3) | 69 (70.4) | 71 (61.2) | 0.592 |
| Unstable angina | 39 (10.1) | 10 (12.0) | 10 (11.0) | 7 (7.1) | 12 (10.3) | 0.564 |
| NSTEMI | 68 (17.5) | 14 (16.9) | 12 (13.2) | 17 (17.3) | 25 (21.6) | 0.247 |
| STEMI | 24 (6.2) | 6 (7.2) | 5 (5.5) | 5 (5.1) | 8 (6.9) | 0.958 |
Data presented as number (percentage), median (interquartile range), or mean±standard deviation. BMI: body mass index; CABG: coronary artery bypass grafting; CCS: chronic coronary syndromes; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate using the Modification of Diet in Renal Disease study formula; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; Pt.: patients; STEMI: ST-segment elevation myocardial infarction; TIA: transient ischemic attack.
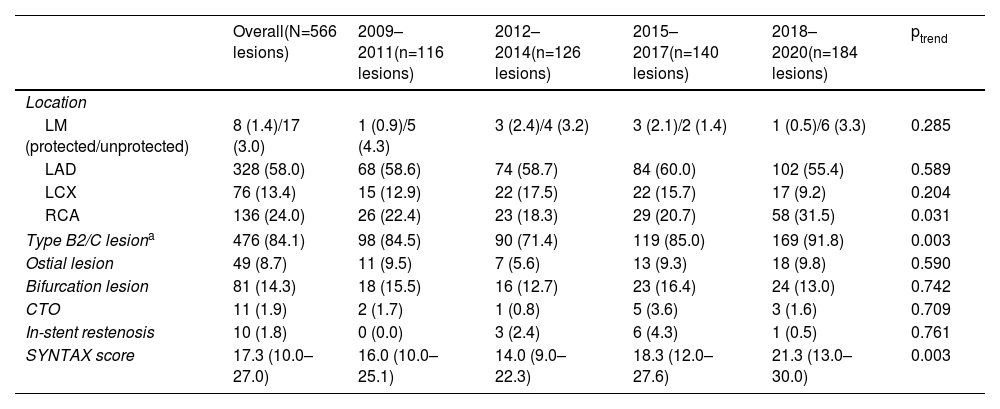
Multivessel disease was predominant, with 149 patients (38.4%) having two-vessel and 127 three-vessel CAD (32.7%). Significant left main disease (i.e., ≥50% stenosis) was present in 50 patients (12.2%). CAD complexity increased over time, including median SYNTAX (Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery) score (ptrend=0.003) and proportion of American College of Cardiology/American Heart Association types B2 and C lesions (up to 91.8%, ptrend=0.003). Table 2 summarizes the target lesion characteristics.
Temporal trends in target lesion characteristics by three-year intervals.
| Overall(N=566 lesions) | 2009–2011(n=116 lesions) | 2012–2014(n=126 lesions) | 2015–2017(n=140 lesions) | 2018–2020(n=184 lesions) | ptrend | |
|---|---|---|---|---|---|---|
| Location | ||||||
| LM (protected/unprotected) | 8 (1.4)/17 (3.0) | 1 (0.9)/5 (4.3) | 3 (2.4)/4 (3.2) | 3 (2.1)/2 (1.4) | 1 (0.5)/6 (3.3) | 0.285 |
| LAD | 328 (58.0) | 68 (58.6) | 74 (58.7) | 84 (60.0) | 102 (55.4) | 0.589 |
| LCX | 76 (13.4) | 15 (12.9) | 22 (17.5) | 22 (15.7) | 17 (9.2) | 0.204 |
| RCA | 136 (24.0) | 26 (22.4) | 23 (18.3) | 29 (20.7) | 58 (31.5) | 0.031 |
| Type B2/C lesiona | 476 (84.1) | 98 (84.5) | 90 (71.4) | 119 (85.0) | 169 (91.8) | 0.003 |
| Ostial lesion | 49 (8.7) | 11 (9.5) | 7 (5.6) | 13 (9.3) | 18 (9.8) | 0.590 |
| Bifurcation lesion | 81 (14.3) | 18 (15.5) | 16 (12.7) | 23 (16.4) | 24 (13.0) | 0.742 |
| CTO | 11 (1.9) | 2 (1.7) | 1 (0.8) | 5 (3.6) | 3 (1.6) | 0.709 |
| In-stent restenosis | 10 (1.8) | 0 (0.0) | 3 (2.4) | 6 (4.3) | 1 (0.5) | 0.761 |
| SYNTAX score | 17.3 (10.0–27.0) | 16.0 (10.0–25.1) | 14.0 (9.0–22.3) | 18.3 (12.0–27.6) | 21.3 (13.0–30.0) | 0.003 |
Data presented as number (percentage) or median (interquartile range). CTO: chronic total occlusion; LAD: left anterior descending artery; LCX: left circumflex artery; LM: left main coronary artery; RCA: right coronary artery; SYNTAX: Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery.
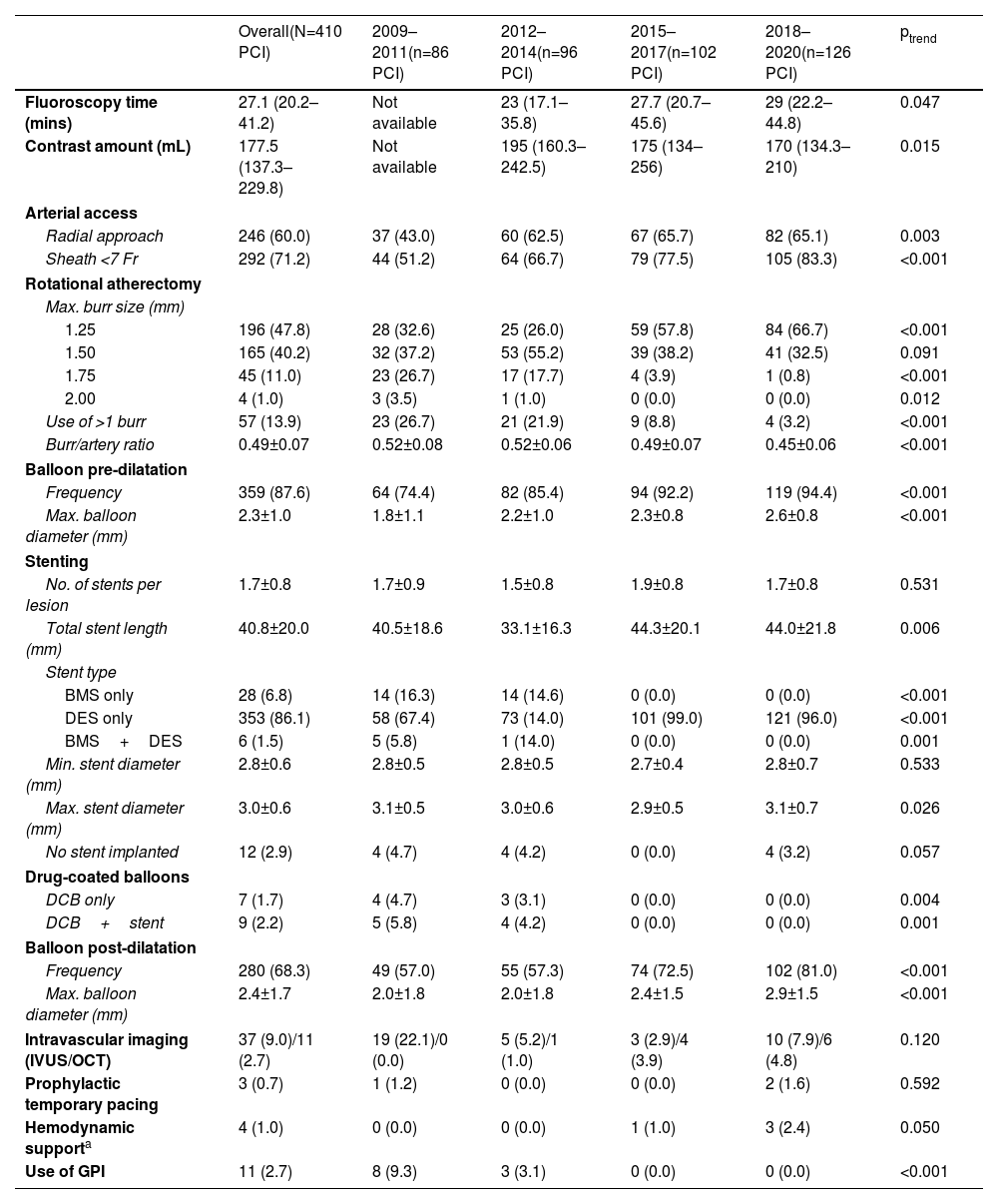
Procedural characteristics and their temporal changes are presented in Table 3. RA was employed upfront in 324 cases (79.0%), staged in 22 cases (5.4%) and performed as an ad hoc bailout strategy in the remainder. Reasons for crossover to RA were the inability to cross the lesion with a balloon or stent (67.7%), inadequate balloon expansion (28.1%) and balloon rupture (3.1%).
Temporal trends in procedural characteristics at three-year intervals.
| Overall(N=410 PCI) | 2009–2011(n=86 PCI) | 2012–2014(n=96 PCI) | 2015–2017(n=102 PCI) | 2018–2020(n=126 PCI) | ptrend | |
|---|---|---|---|---|---|---|
| Fluoroscopy time (mins) | 27.1 (20.2–41.2) | Not available | 23 (17.1–35.8) | 27.7 (20.7–45.6) | 29 (22.2–44.8) | 0.047 |
| Contrast amount (mL) | 177.5 (137.3–229.8) | Not available | 195 (160.3–242.5) | 175 (134–256) | 170 (134.3–210) | 0.015 |
| Arterial access | ||||||
| Radial approach | 246 (60.0) | 37 (43.0) | 60 (62.5) | 67 (65.7) | 82 (65.1) | 0.003 |
| Sheath <7 Fr | 292 (71.2) | 44 (51.2) | 64 (66.7) | 79 (77.5) | 105 (83.3) | <0.001 |
| Rotational atherectomy | ||||||
| Max. burr size (mm) | ||||||
| 1.25 | 196 (47.8) | 28 (32.6) | 25 (26.0) | 59 (57.8) | 84 (66.7) | <0.001 |
| 1.50 | 165 (40.2) | 32 (37.2) | 53 (55.2) | 39 (38.2) | 41 (32.5) | 0.091 |
| 1.75 | 45 (11.0) | 23 (26.7) | 17 (17.7) | 4 (3.9) | 1 (0.8) | <0.001 |
| 2.00 | 4 (1.0) | 3 (3.5) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0.012 |
| Use of >1 burr | 57 (13.9) | 23 (26.7) | 21 (21.9) | 9 (8.8) | 4 (3.2) | <0.001 |
| Burr/artery ratio | 0.49±0.07 | 0.52±0.08 | 0.52±0.06 | 0.49±0.07 | 0.45±0.06 | <0.001 |
| Balloon pre-dilatation | ||||||
| Frequency | 359 (87.6) | 64 (74.4) | 82 (85.4) | 94 (92.2) | 119 (94.4) | <0.001 |
| Max. balloon diameter (mm) | 2.3±1.0 | 1.8±1.1 | 2.2±1.0 | 2.3±0.8 | 2.6±0.8 | <0.001 |
| Stenting | ||||||
| No. of stents per lesion | 1.7±0.8 | 1.7±0.9 | 1.5±0.8 | 1.9±0.8 | 1.7±0.8 | 0.531 |
| Total stent length (mm) | 40.8±20.0 | 40.5±18.6 | 33.1±16.3 | 44.3±20.1 | 44.0±21.8 | 0.006 |
| Stent type | ||||||
| BMS only | 28 (6.8) | 14 (16.3) | 14 (14.6) | 0 (0.0) | 0 (0.0) | <0.001 |
| DES only | 353 (86.1) | 58 (67.4) | 73 (14.0) | 101 (99.0) | 121 (96.0) | <0.001 |
| BMS+DES | 6 (1.5) | 5 (5.8) | 1 (14.0) | 0 (0.0) | 0 (0.0) | 0.001 |
| Min. stent diameter (mm) | 2.8±0.6 | 2.8±0.5 | 2.8±0.5 | 2.7±0.4 | 2.8±0.7 | 0.533 |
| Max. stent diameter (mm) | 3.0±0.6 | 3.1±0.5 | 3.0±0.6 | 2.9±0.5 | 3.1±0.7 | 0.026 |
| No stent implanted | 12 (2.9) | 4 (4.7) | 4 (4.2) | 0 (0.0) | 4 (3.2) | 0.057 |
| Drug-coated balloons | ||||||
| DCB only | 7 (1.7) | 4 (4.7) | 3 (3.1) | 0 (0.0) | 0 (0.0) | 0.004 |
| DCB+stent | 9 (2.2) | 5 (5.8) | 4 (4.2) | 0 (0.0) | 0 (0.0) | 0.001 |
| Balloon post-dilatation | ||||||
| Frequency | 280 (68.3) | 49 (57.0) | 55 (57.3) | 74 (72.5) | 102 (81.0) | <0.001 |
| Max. balloon diameter (mm) | 2.4±1.7 | 2.0±1.8 | 2.0±1.8 | 2.4±1.5 | 2.9±1.5 | <0.001 |
| Intravascular imaging (IVUS/OCT) | 37 (9.0)/11 (2.7) | 19 (22.1)/0 (0.0) | 5 (5.2)/1 (1.0) | 3 (2.9)/4 (3.9) | 10 (7.9)/6 (4.8) | 0.120 |
| Prophylactic temporary pacing | 3 (0.7) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 2 (1.6) | 0.592 |
| Hemodynamic supporta | 4 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 3 (2.4) | 0.050 |
| Use of GPI | 11 (2.7) | 8 (9.3) | 3 (3.1) | 0 (0.0) | 0 (0.0) | <0.001 |
Data presented as number (percentage), median (interquartile range), or mean±standard deviation. BMS: bare-metal stent; DCB: drug-coated balloon; DES: drug-eluting stent; GPI: glycoprotein IIb/IIIa inhibitors; IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; RA: rotational atherectomy.
Fluoroscopy time increased (ptrend=0.047) and the amount of contrast dye declined (ptrend=0.015) throughout the study period. Transradial approach and the use of <7Fr sheaths increased significantly over time (ptrend=0.003 and ptrend <0.001, respectively). Regarding rotablation, the use of burr diameters over 1.25 mm decreased over time (ptrend <0.001) in parallel with a slight reduction in burr/artery ratio, from 0.52±0.08 to 0.45±0.06 (ptrend <0.001). A single burr was sufficient in most cases (86.1%), with the employment of more than one burr being residual in the last triennium (3.2%).
Intravascular imaging was roughly used in one out of ten procedures; an increase in optical coherence tomography utilization was observed up to 4.8% in the last three years. In 63 cases (15.4%), other calcium modifying tools were employed along with RA, specifically cutting balloons (n=60), scoring balloons (n=2), and intravascular lithotripsy (n=1).
A total of 700 stents were implanted, 92.6% of which were DES (100% after 2014, ptrend <0.001). High-pressure post-dilatation was undertaken in 68.3%, with growing frequency over time, reaching 81% in the last three years (ptrend <0.001). A similar growing trend was observed in terms of total stent length, rising to 44.0±21.8 mm (ptrend=0.006). Glycoprotein IIb/IIIa inhibitors were seldom used overall and never administered from 2013 onwards (ptrend <0.001). Prophylactic temporary pacing and hemodynamic support were exceptional (three and four procedures, respectively).
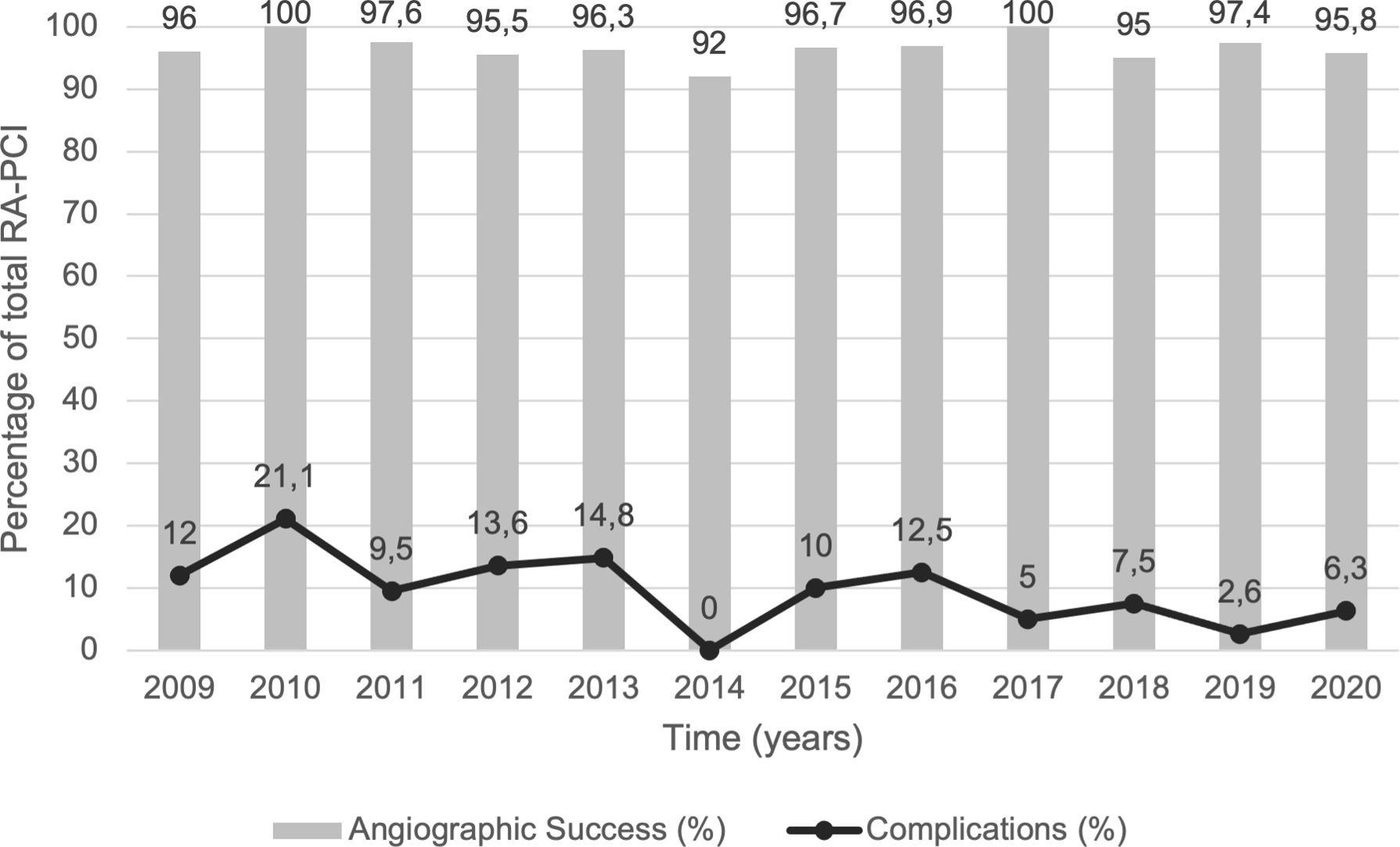
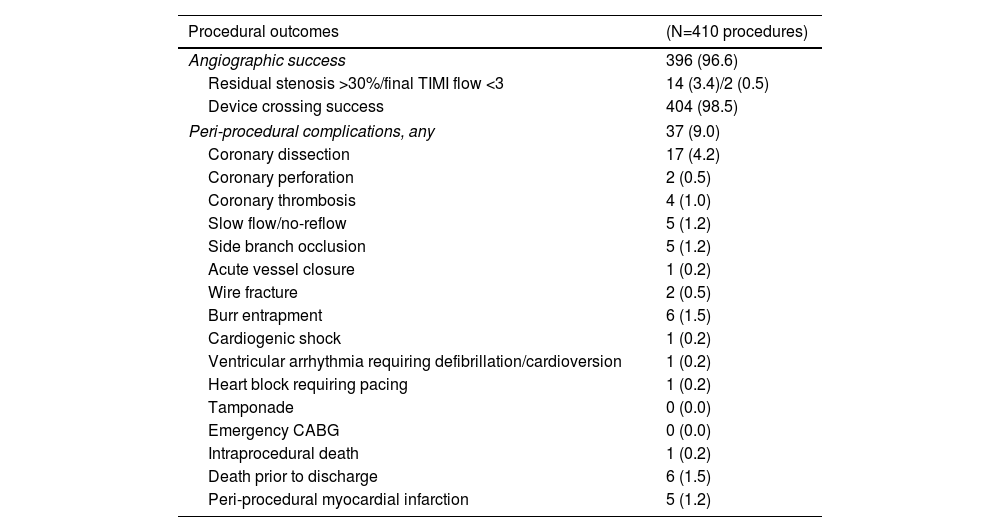
OutcomesEarly and long-term outcomes are detailed in Table 4. The angiographic success rate was consistently high throughout the study period (96.6% overall; ptrend=0.938; Figure 2). Despite several runs with the smallest burr, six lesions remained uncrossable, deeming those procedures unsuccessful. Peri-procedural complications were recorded in 37 procedures (9.0%), with a downward trend over time (ptrend=0.029; Figure 2). The most common complication was coronary dissection, which generally required additional stenting (82% of dissections). Six deaths (1.5%) occurred prior to hospital discharge, one of which during the procedure (cardiac arrest following acute coronary syndrome presentation requiring PCI to the proximal left anterior descending artery). All cases of burr entrapment and perforation were successfully managed percutaneously. Univariate analysis identified acute coronary syndrome at presentation and bailout RA as predictors of peri-procedural complications, yet only bailout RA remained significant in the multivariate model (OR: 3.60; 95% CI: 1.55–8.35; p=0.003; Table S1).
Early and long-term outcomes.
| Procedural outcomes | (N=410 procedures) |
|---|---|
| Angiographic success | 396 (96.6) |
| Residual stenosis >30%/final TIMI flow <3 | 14 (3.4)/2 (0.5) |
| Device crossing success | 404 (98.5) |
| Peri-procedural complications, any | 37 (9.0) |
| Coronary dissection | 17 (4.2) |
| Coronary perforation | 2 (0.5) |
| Coronary thrombosis | 4 (1.0) |
| Slow flow/no-reflow | 5 (1.2) |
| Side branch occlusion | 5 (1.2) |
| Acute vessel closure | 1 (0.2) |
| Wire fracture | 2 (0.5) |
| Burr entrapment | 6 (1.5) |
| Cardiogenic shock | 1 (0.2) |
| Ventricular arrhythmia requiring defibrillation/cardioversion | 1 (0.2) |
| Heart block requiring pacing | 1 (0.2) |
| Tamponade | 0 (0.0) |
| Emergency CABG | 0 (0.0) |
| Intraprocedural death | 1 (0.2) |
| Death prior to discharge | 6 (1.5) |
| Peri-procedural myocardial infarction | 5 (1.2) |
| Long-term outcomes | (N=357 patients) |
|---|---|
| MACE | 99 (27.7) |
| Cardiovascular deatha | 73 (20.4) |
| Myocardial infarction | 27 (7.6) |
| Target lesion revascularization | 27 (7.6) |
| All-cause mortality | 114 (31.9) |
| Non cardiovascular death | 41 (11.5) |
| Undetermined | 36 (10.1) |
| Target vessel revascularization | 35 (9.8) |
| Definite stent thrombosis | 4 (1.1) |
| Heart transplant | 1 (0.3) |
Data presented as number (percentage) or median (interquartile range). CABG: coronary artery bypass grafting; MACE: major adverse cardiovascular events; TIMI: thrombolysis in myocardial infarction.
Trends in peri-procedural outcomes over the study period (2009–2020). Angiographic success rate (grey bars) remained unchanged over time (ptrend=0.938), while the incidence of peri-procedural complications (black line) declined (ptrend=0.029). RA-PCI: percutaneous coronary intervention with rotational atherectomy.
Follow-up data were available for 357 patients (92.0%). The incidence of MACE was 27.7% at a median follow-up period of 40 (16–76) months and was mainly driven by cardiovascular death (20.4%). The rate of all-cause death was 31.9%. At one year, MACE, all-cause mortality, and target vessel revascularization occurred in 12.1%, 6.4%, and 5.6% of patients, respectively, with no significant changes during the study period. Multivariable risk adjustment confirmed this finding (Table S2). Chronic kidney disease (HR: 1.98; 95% CI: 1.05–3.742; p=0.035) and multivessel disease (HR: 2.58; 95% CI: 1.09–6.126; p=0.032; Table S3) were significantly associated with the occurrence of MACE during follow-up.
DiscussionThis single-centre cohort analysis explored the temporal trends and outcomes of RA-PCI. Our main findings were: (a) the rotablation/PCI ratio is recently rising, with some technical modifications; (b) the angiographic success was consistently high; (c) peri-procedural complications were infrequent, rarely severe, and declined during the study period; (d) in this high-risk group the rate of follow-up MACE was acceptable and did not change over time.
Adequate lesion preparation is paramount to PCI success in severely calcified lesions. Currently, there are plentiful options for this purpose: specialty balloons (cutting, scoring, and ultra-high pressure), intravascular lithotripsy, and atherectomy (rotational, orbital, and laser). However, the lack of well-defined criteria makes device selection challenging.23 RA has consistently shown excellent procedural results, though it is unclear whether they translate into long-term benefit, as the contemporary RA trials were underpowered to detect differences in such outcomes.16,17
Our institution's RA-PCI rate of 2.8% falls within the 1–3% range reported in national and international registries.18,24–26 Interestingly, the ratio was highest in 2011–2012 after contemporaneous guidelines granted a Class I (level of evidence C) recommendation to rotablation,27 but decreased substantially in the following year, possibly due to the discouraging results of the ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial, that failed to show superiority of an RA-based strategy versus standard therapy before DES implantation.16 Nevertheless, the RA rate is increasing again, particularly since the publication of the European Experts Consensus15 and the PREPARE-CALC (Comparison of Strategies to Prepare Severely Calcified Coronary Lesions) trial.17
In most cases, RA was planned beforehand. However, in 15.6% of our procedures, ad hoc bailout RA was necessary to treat uncrossable or undilatable lesions, a low rate compared to the ROTATE registry (approximately half of the cases)28 and a report from Abdel-Wahab et al. (27%).29
Regarding baseline characteristics, the high-risk patient profile is noteworthy. Consistently with previous reports, our results showed that RA patients are elderly with a high burden of cardiovascular risk factors and co-morbidities, suggesting an increased baseline predisposition for adverse events.18,30–32 Likewise, especially in recent years, the high proportion of B2/C lesions, the median SYNTAX score, and total stent length were striking, reflecting the increasingly high CAD complexity of this patient subset.24
Rotational atherectomy is relatively contraindicated in acute coronary syndromes because of the risk of further platelet activation in an inherently thrombogenic environment.33 However, calcified lesions are common among these patients and balloon dilation is often insufficient to achieve procedural success. That was the case for almost a third of our patients, that required RA to either treat the culprit or achieve complete revascularization. Some observational data demonstrated that RA is feasible and safe in acute coronary syndrome but associated with worse long-term outcomes.34 Further studies are warranted to confirm these findings.
Concerning the procedural details, some technical modifications were noticed. Over time, a less aggressive plaque modification strategy was adopted (fewer and smaller burrs were used), more patients underwent high-pressure post-dilatation, and the transradial approach became the preferential access route. This is in line with the shift observed between the ROTATE registry (2002–2013) and the Euro4C registry (2016–2018), and presumably reflects the change in the overall standard of care, as well as the decreased use of larger burrs, allowing for the use of smaller calibre sheaths and guiding catheters and, consequently, radial access.30,31
Although our use of intravascular imaging is somewhat higher than that of other European centres (e.g., 11.7% vs 6.9% in the Euro4C registry31), it is still surprisingly low considering the complexity of these cases. Possible reasons for the low intracoronary imaging use include: long procedure time, need to limit contrast agent use (almost a third of our patients had chronic kidney disease), difficult access to and navigation through the often small, tortuous vessels, cost and resource constraints, etc. Increasing the use of intravascular imaging may represent an opportunity to improve outcomes by allowing adequate stent sizing and prompting further plaque modification or stent optimization through post-dilatation.
Peri-procedural outcomesAngiographic success rate was high (96.6% in 410 cases) in support of previous studies, despite variation in success definition.16–18,31 Even though RA has been recognized as an important risk factor for coronary perforation,35 only two cases were recorded in our cohort. Overall, we found a relatively low and declining rate of peri-procedural complications, which may reflect the learning curve,32 as well as the strict compliance with current best practices: use of small burrs (maximum burr size of <1.75 mm in 88.0% of our cases; burr-to-artery ratio of 0.49±0.07), rotational speed <180000 rpm, pecking burr motion, short ablation runs, and avoidance of abrupt decelerations.15
While crossing over to RA can enable procedural success, this approach may increase fluoroscopy time, contrast medium volume, and complication rates.28,36 Indeed, a bailout strategy was an independent predictor of peri-procedural complications in our cohort. This could reflect the risk of extending pre-existing dissections caused by repeated high-pressure pre-dilatation or specialty balloon use. Further studies are needed to determine if a staged RA approach would be superior in this setting to allow “healing” of such dissections,28 which is not an uncommon strategy in our practice.
Long-term outcomesTheoretically, RA can act both in favour and against DES. On the one hand, RA smoothens the lumen and helps to fracture calcium rings, facilitating lesion crossing with minimal polymer disruption, as well as optimal stent expansion and apposition. On the other hand, thermal injury and mechanical vessel trauma may trigger an exaggerated neointimal response and increase restenosis.37 In our cohort, follow-up MACE was 27.2% at 40 months and was mainly driven by cardiovascular death (20.4%), as reported in the Euro4C registry for one-year MACE.31 Chronic kidney disease and multivessel disease were significant predictors of MACE. Due to the exploratory and retrospective nature of long-term outcomes analysis, only 92.0% of the cohort (80.8% at one year) had complete follow-up data. As a referral centre, our institutional records are more likely to be updated in patients with recurrent events/interventions, therefore adverse clinical outcomes may be overestimated. In light of the high clinical complexity and this information bias, the MACE rate is acceptable.
LimitationsThis analysis has some limitations. First, data quality may be suboptimal due to missing data and heterogeneity among interventional team members introducing the data. Second, we were unable to account for all potential confounding factors, including those prompting planned RA employment, as we lack information on non-RA procedures. Third, due to the low number of outcomes, high uncertainty is brought into the multivariable logistic regression on peri-procedural complications, demonstrated by the broad CI of predictor variables. Fourth, the incidence of peri-procedural MI was lower than previously reported,30 due to the lack of data to retrospectively adjudicate this outcome. Lastly, the true effect of the bias created by the missing follow-up data is hard to estimate, but possibly would drive the MACE rate down.
The retrospective observational design of this study makes it prone to bias, thus demanding careful interpretation and limiting data extrapolation. However, the lack of exclusion criteria offered the opportunity to characterize an all-comers population undergoing RA, providing a comprehensive evaluation of the utilization and safety of this device in routine clinical practice.
ConclusionsIn this single-centre all-comers analysis, RA was safe and effective with a high rate of procedural success. Over time, less and smaller burrs were used, employing smaller calibre sheaths, high-pressure post-dilatation was undertaken more frequently, and the radial approach became the preferred access route, with lower complications’ rate. The incidence of MACE is acceptable considering patient and CAD complexity.
FundingThe authors declare no financial relationships.
Conflicts of interestThe authors declare no conflicts of interest.