We report the case of a 17-year-old athlete who resorted to the emergency department for palpitations and dizziness while exercising. He mentioned two exercise-associated episodes of syncope in the last six months. He was tachycardic and hypotensive. The electrocardiogram showed regular wide complex tachycardia, left bundle branch block morphology with superior axis restored to sinus rhythm after electrical cardioversion. In sinus rhythm, it showed T-wave inversion in V1–V5. Transthoracic echocardiography revealed mild dilation and dysfunction of the right ventricle (RV) with global hypocontractility. Cardiac magnetic resonance (CMR) revealed a RV end diastolic volume indexed to body surface area of 180 ml/m2, global hypokinesia and RV dyssynchrony, subepicardial late enhancement in the distal septum and in the middle segment of the inferoseptal wall. The patient underwent a genetic study which showed a mutation in the gene that encodes the desmocolin-2 protein (DSC-2), which is involved in the pathogenesis of arrhythmogenic right ventricular cardiomyopathy (ARVC). According to the modified Task Force Criteria for this diagnosis, the patient presented four major criteria for ARVC. Thus, a subcutaneous cardioverter was implanted, and the patient was followed up at the cardiology department.
Arrhythmogenic right ventricular cardiomyopathy diagnosis is based on structural, functional, electrophysiological and genetic criteria reflecting underlying histological changes. This case depicts the essential characteristics for disease recognition.
Reportamos o caso de um jovem desportista de 17 anos que recorreu ao serviço de urgência por palpitações e tonturas durante o exercício físico. Referia dois episódios de síncope com o exercício nos últimos seis meses. Na admissão apresentava-se taquicárdico e hipotenso. O eletrocardiograma mostrava taquicardia regular de complexos largos com morfologia de bloqueio completo de ramo esquerdo e eixo superior, com necessidade de cardioversão elétrica. Em ritmo sinusal evidenciava inversão da onda T de V1-V5. O ecocardiograma transtorácico demonstrava dilatação e disfunção ligeira do ventrículo direito (VD) por hipocontratilidade global. A RMC revelava um volume telediastólico do VD indexado para a superfície de área corporal de 180 ml/m2, hipocinésia global e dessincronia do VD, realce tardio subepicárdico no septo distal e no segmento médio da parede infero-septal. O doente realizou estudo genético que mostrou mutação no gene DSC2 que codifica a proteína desmocolina-2 implicada na patogénese da miocardiopatia arritmogénica do ventrículo direito (MAVD). De acordo com os critérios Task Force modificados de 2010, o doente apresentava quatro critérios major para o diagnóstico de MAVD. O doente implantou cardiodesfibrilhador subcutâneo, encontrando-se em seguimento na consulta de cardiologia.
O diagnóstico de MAVD assenta em critérios estruturais, funcionais, genéticos e eletrofisiológicos que traduzam as alterações histológicas subjacentes. Este caso reflete as características essenciais ao seu reconhecimento.
Sudden cardiac death (SCD) in an athlete is a rare event with an incidence ranging from 1:50000 to 1:200000 athletes per year.1 Cardiovascular screening in athletes prior to exercise is recommended by both the American Heart Association and the European Society of Cardiology.2,3 In addition to clinical history and physical examination, the ESC recommends electrocardiogram (ECG) as a screening method because it is an available and inexpensive test that shows alterations in >80% of the cases of myocardiopathies, myocarditis, pre-excitation syndrome and channelopathies responsible for most SCDs in athletes <35 years of age.3
Exercise-triggered syncope is an alarming symptom because of its relationship to heart disease – although it may sometimes be related to exaggerated reflex vasodilator activity – and should alert us to the need to conduct more robust studies that rule out cardiac disease.4,5 Most of the studies related to the etiology of SCD in young athletes are limited by the lack of post-mortem data, and therefore there is no full consensus among the various registries.1,6 While in some studies, myocardiopathies seem to predominate, others suggest the predominance of primary arrhythmic syndromes, such as Brugada syndrome or long QT syndrome, which account for approximately one third of sudden arrhythmic death syndromes.1,6,7 Within myocardial diseases, idiopathic left ventricular (LV) hypertrophy/idiopathic fibrosis and arrhythmogenic right ventricular cardiomyocardiopathy (ARVC) seem to be of particular relevance.1,6 ARVC has a prevalence of 1 in 1000–5000 people, accounting for 20% of cases of SCD.4,8 This disease is usually inherited, with an autosomal dominant transmission pattern, and is histologically characterized by the replacement of myocardial tissue with fibroadipose tissue in the so-called “triangle of dysplasia” of the right ventricle (RV) – inflow tract, outflow tract, and RV apex – although progression to diffuse RV and LV involvement is frequent (up to 50%), particularly in the posterolateral wall.4,9,10 Its diagnosis is based on structural, functional, genetic and electrophysiological criteria. The first International Task Force published in 1994 proposed a set of criteria for the diagnosis of ARVC derived from clinical experience, which had been until then based primarily on symptomatic index cases and on victims of SCD. These criteria were considered highly specific, but demonstrated low sensitivity over time for the diagnosis of cases with mild phenotypes.11 Thus, in order to improve the diagnostic capability of these criteria, in 2010 the modified Task force Criteria (TFC) were proposed, emphasizing the emerging role of new imaging modalities and advances in the genetic study of ARVC, maintaining their diagnostic specificity but increasing their sensitivity.10 The case we present is a paradigmatic example of this change.
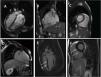
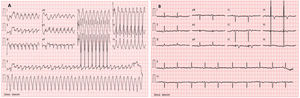
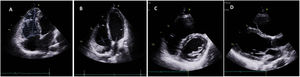
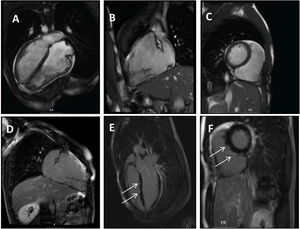
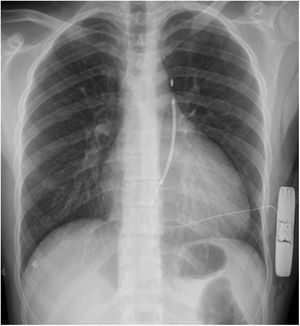
Clinical caseWe report the case of a 17-year-old federated soccer player who sought emergency care following an episode of palpitations and dizziness while playing soccer. He reported having already had two episodes of syncope upon exertion in the last six months but did not value them and therefore did not go to the emergency room. He denied any other personal history, regular consumption of drugs or narcotics and any relevant family history, including a history of SCD. The patient was tachycardic (218 bpm) and hypotensive (85/56 mmHg). A 12-lead ECG was performed which revealed wide complex tachycardia with left bundle branch block morphology and superior axis (Figure 1). Given the hemodynamic instability, the patient underwent electrical cardioversion with a biphasic shock of 50 joules and reverted to sinus rhythm. The ECG in sinus rhythm showed a T-wave inversion of V1–V5, with no other alterations (Figure 1). From laboratory tests, only a slight elevation of troponin I (0.97 ng/ml) was noteworthy. A transthoracic echocardiogram showed a non-dilated LV with good global systolic function, slight RV dilation and slight depression of its function (FAC) of 29%), with no other changes (Figure 2). The patient was hospitalized and underwent cardiac magnetic resonance imaging (CMR). CMR showed marked RV dilatation (end-diastolic volume indexed to body surface area of 180 ml/m2) by global hypokinesis with mild depression of its function (42%) and desynchrony. The late enhancement images showed subepicardial late enhancement in the distal septum and two small foci of subepicardial late enhancement in the middle segment of the inferoseptal wall (Figure 3). Thus, according to the 2010 TFC for the diagnosis of ARVC, the patient had three major criteria for the diagnosis of ARVC, including morphofunctional changes (areas of hypocontractility with a FAC ≤33%; desynchrony and RV end-diastolic volume indexed to body surface area ≥110 ml/m2), repolarization changes (inverted T-wave of V1–V5 in the absence of criteria for complete right bundle branch block), arrhythmia (sustained ventricular tachycardia with LBBB morphology and superior axis). Thus, taking into account that only two major criteria are needed for its diagnosis, the diagnosis of ARVC was made. Also, during hospitalization, the patient was implanted with a subcutaneous cardioverter-defibrillator (Figure 4). The patient remained asymptomatic throughout hospitalization. He was discharged with low-dose beta-blocker medication and contraindication for practicing sports. He is in regular follow-up in the Cardiology consultation, and adheres to the prescribed medication and recommendations, having, to date, no record of any new arrhythmic events. During follow-up, a genetic test was requested and enabled the identification of a pathogenic mutation categorized as associated to ARVC – variant c.1044_104dupAAAT (p.Asp350Lysfs*2) in the DSC2 gene which encodes the desmocholine-2 protein, and is implicated in the pathogenesis of ARVC. Thus, the patient has four major diagnostic criteria. A Holter was also requested which showed a number of ventricular extrasystoles >500 per day, fulfilling a minor criterion for the diagnosis. Currently, the patient does not participate in high-level sports nor any type of recreational sport.
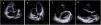
Panel A–C: CINE CMR images showing marked RV dilatation (end diastolic volume indexed to body surface area of 180 ml/m2), global hypokinesis and dyssynchronous RV contractility with mild depression of its function (42%); Panel D–F: late-enhanced CMR showing subepicardial late enhancement in the distal septum and two small foci of subepicardial late enhancement in the middle segment of the inferoseptal wall (arrows).
The diagnosis of ARVC in athletes is particularly challenging, since in the early stages the heart may appear structurally normal. On the other hand, recurrent exercise is associated with a spectrum of morphofunctional and electrical adaptive changes known as “the athlete's heart”, which are usually only modest and within the spectrum of normal.12 Occasionally, some athletes may show marked electrical and structural changes that overlap with changes typical of some cardiac diseases.3,13,14 For example, endurance sports are associated with increased cardiac output, resulting in physiological RV dilation (usually proportional to LV dilation) while maintaining preserved systolic function, in contrast to ARVC in which outflow tract dilation predominates.15 However, when intense exercise is sustained for several hours, there may be degradation of RV function, and its degree appears to be dependent on the length of sustained exercise.13,16
In a meta-analysis published on this topic, virtually all studies assessing RV function after prolonged intense endurance exercise identified significant RV dysfunction.16 Thus, in some cases there may be some overlap between the structural diagnostic criteria for RVMD and exercise-associated cardiac changes. This theory was corroborated by Heidbuchel et al. who suggested that athletes exposed to extreme exercise may develop RV structural changes compatible with ARVC, regardless of their genetic risk. In their study, a recurrent clinical pattern of ventricular arrhythmias originating in RV and RV dysfunction was observed in professional cyclists with palpitations. In fact, 85% and 89% of these athletes met the diagnosis for ARVC or probable ARVC, even though they had a negative genetic study.13 This “exercise-induced RV myocardiopathy”, clinically similar to ARVC, seems to be caused by exercise-associated hemodynamic stress and consecutive peaks of RV dilation and dysfunction with consequent alteration of its extracellular matrix rather than a genetic predisposition.
The exercise-induced RV myocardiopathy hypothesis explains the low prevalence of desmosome gene mutations in endurance athletes with complex arrhythmias originating from the RV.13,14 Studies are needed to validate the normal physiological RV limits in athletes, as studies to establish RV size in modified TFC were based on comparing patients with ARVC with a healthy control group that did not include athletes. In fact, more than 30 to 60% of athletes have RV sizes that meet major and minor criteria for ARVC, respectively.15 Therefore, in athletes RV dilatation should be suggestive of ARVC only when extreme (V outflow tract diameter indexed to body surface area (BSA) on the long axis >19 mm/m2 and/or >21 mm/m2 on the short axis and/or RV end-diastolic volume (EDV) indexed to BSA in CMR ≥110 ml/m2 in men and ≥100 ml/m2 in women) and if associated with segmental motility changes.3 Echocardiographic ratio of RV inflow tract dimension (apical) under LV end-diastolic dimension (in long axis) >0.9 or RV EDV/LV EDV >1.2 on CMR are also shown to have specificity for the diagnosis of ARVC in athletes.10 CMR plays a key role in the diagnostic approach to ARVC and is crucial for establishing the diagnosis, since it allows for a highly detailed assessment of RV function and morphology.3 Standard protocols for the evaluation of ARVC by CMR include cine imaging to assess cardiac function, T1-weighted (dark blood) imaging with and without fat saturation to assess ventricular morphology, and late gadolinium enhancement to study myocardial fibrosis, enabling qualitative assessment of segmental motility and global quantification of RV size and function. Quantitative assessment was added in the 2010 revision, limiting diagnostic subjectivity and increasing specificity.10
Luijkc et al. compared dimensions, volumes and function of the RV via CMR in 33 patients with ARVC and healthy athletes and nonathletes, concluding that the modified TFC enables a distinction to be made between individuals with ARVC and athletes with adaptive changes to exercise, since they showed no real changes in segmental kinetics, an indispensable criterion of the modified TFC.17 In the presence of segmental kinetic changes, quantitative criteria for RV ejection fraction (EF) were applied to distinguish ARVC from physiological changes in athletes.3,17 However, in the absence of segmental kinetic changes, more athletes met the criteria for ARVC according to the cut-off established for the indexed RVOT of the modified TFC than true ARVC patients.3,17
As an alternative volume criterion, the previously described ratio (RV EDV/LV EDV) was proposed as a better discriminator of an athlete's adaptive changes from pathological changes in ARVC.3 Although LV involvement is not uncommon in the progression of ARVC, RV dilation is usually disproportionate to that of the LV, so larger RV EDV/LV EDV ratios have been identified in individuals with ARVC compared to athletes’ hearts. In conclusion, if an abnormality of segmental kinetics is present, then RV EF can help distinguish between ARVC and physiological changes in the athlete. Otherwise, a good alternative in athletes may be to use the RVOT/RVOT ratio.3
Even in the era of state-of-the-art technology, the relevance of ECG changes in the diagnosis of ARVC remains unwavering. In 1994, ECG changes were considered a minor criterion.4,10 However, characteristic ECG abnormalities are present in most patients who comply with other TFC.18 Thus, in 2010, typical AVRD changes, namely T-wave inversion of V1–V3 or more in individuals >14 years in the absence of criteria for complete right bundle branch block, became a major criterion for the disease.10 These electrical changes should be placed in the context of imaging findings. Minor electrocardiographic abnormalities in the right leads, rare ventricular extrasystoles with morphology compatible with RV origin, and subtle changes in the RV may be the only objective abnormalities of the disease, which overlap with physiological changes adapting to exercise.19 However, their association with the presence of epsilon waves, repolarization changes in right precordial leads, low amplitude QRS complexes in right precordial leads, ventricular tachycardia with morphology of complete left bundle branch block (LBBB), frequent ventricular extrasystoles on Holter (>500 in 24 hours), evidence of late potentials or positive electrophysiological study support the diagnosis of ARVC.10,18
Only in a minority of cases is the disease related to mutations in nonmosomal genes and recessive forms of transmission, such as Carvajal syndrome and Naxos disease, which are associated with palmoplantar hyperkeratosis.4,10 A positive genetic study should be part of a broad diagnostic protocol involving the already described diagnostic methods and criteria. DSC2 gene mutations account for only 5% of ARVC cases.10,19 Most are heterozygous mutations leading to the typical forms of ARVC, whereas homozygous forms can lead either to phenotypes restricted to cardiac changes or to phenotypes with both cardiac and cutaneous alterations.10
Syncope and sudden cardiac death in athletesThe presence of syncope in athletes represents a unique diagnostic challenge. This may be the first manifestation of several serious diseases, such as hypertrophic cardiomyopathy (HCM), ARVC, anomalous coronary artery origin, myocarditis, Brugada syndrome or long QT syndrome.20,21 In individuals with a disease with an arrhythmic substrate, catecholaminergic release spikes, dehydration, hyperpyrexia, and hydroelectrolyte imbalances during intense exercise may contribute to the induction of ventricular tachycardia phenomena and SCD.20 There are few epidemiological data available on the etiology of syncope in athletes. A study published in 2004 that studied 7568 young athletes over a five-year period documented at least one episode of syncope in 474 of the athletes, with 63 of these episodes related to physical exercise (57 episodes after exercise and 6 during exercise). Of the six patients with syncope during exercise, one had HCM, one had RV outflow tract tachycardia, and the rest had only a positive response on the Tilt test. No SCD events were detected.20 Despite being a tragic event with a significant impact on the general population, SCD is a relatively rare event, and there is much discussion about cost-effective screening methods to be applied in this population.22
The initial evaluation of an athlete with syncope should include a clinical history with a description of the event that enables its clear distinction from other conditions characterized by an altered state of consciousness. Other data to be checked in the clinical history include the circumstances in which syncope occurs, since syncope during exercise is more often related to the presence of cardiac pathology and SCD than post-exercise syncope, which correlates more often with vasodilation and hypotension phenomena. The 12-lead ECG is also a test that is considered essential in the context of studying syncope. In cases where any of the previous methods reveal changes, the study should proceed to other non-invasive and invasive tests.4
An Italian prospective study revealed an incidence of SCD of about 2.3 cases per 100000 athletes per year from all causes and about 2.1 per 100000 athletes from cardiovascular causes.20,22 In athletes >35 years, atherosclerotic coronary artery disease is the most frequent etiology of SCD, but this scenario changes when it comes to younger athletes, in whom other hereditary or acquired cardiac abnormalities predominate.4 Data from a UK Regional Register studying 357 athletes with SCD under <35 years showed that most cases occurred in athletes with structurally normal hearts due to sudden arrhythmic death syndrome (42% of cases).6 The electrical changes that are most associated with this syndrome are long QT syndrome, Wolff-Parkinson-White syndrome and Brugada syndrome. About 40% of the subjects studied had myocardial disease, including cases of idiopathic left ventricular hypertrophy/fibrosis, SCD, and ARVC.6 HCM is the leading SCD-associated myocardiopathy in athletes, accounting for more than one third of these deaths in the US.21 In the same British registry, the vast majority of SCD in athletes occurred during exercise (61%) and the presence of ARVC was the strongest predictor of risk of SCD during exercise.23 More specifically, athletes with ARVC showed a six times higher risk of death during exercise than the risk associated with other diseases.23 LV fibrosis has also been shown to be an independent predictor of the risk of SCD during exercise.15 Italian data suggest that the risk of SCD is 2.8 times higher in top-level athletes with a cardiac anomaly compared to the same non-athlete patients; this number rises to five times higher for those with ARVC.4 Thus, patients with unequivocal or probable ARVC should be advised not to participate in competitive sports and to limit their exercise to leisure activities (Class IIa, level of evidence C). This recommendation extends to individuals who carry recognized pathogenic mutations, even if they are not phenotypically positive.2,3
ConclusionSyncope in athletes may be the first manifestation of ventricular arrhythmias in the context of potentially fatal cardiomyopathies or channelopathies. This case demonstrates the thoroughness with which screening for heart disease should be approached in symptomatic athletes and illustrates the essential role of cardiac imaging methods, particularly CMR in this process. This case also reminds us of the need to stop all competitive physical activity in patients with ARVC, leading to further discussions on the role of exercise in physiological RV remodeling and exercise-induced RV myocardiopathy.
Conflicts of interestThe authors have no conflicts of interest to declare.