Patients successfully resuscitated from cardiac arrest (CA) are admitted to the intensive care unit (ICU) for post-resuscitation care. These patients’ prognosis remains dismal, with only a minority surviving to hospital discharge. Understanding the clinical factors involved in the management of these patients is essential to improve their prognosis.
ObjectivesTo characterize the population admitted after successful reanimation from CA, and to analyze the factors associated with their outcomes.
MethodsWe performed a retrospective descriptive study of patients admitted to an ICU after CA over a five-year period from January 2014 to December 2018. Demographic factors, CA characteristics, early management, mortality and neurologic outcomes were analyzed.
ResultsA total of 187 patients, median age 67 years, were admitted after CA, of whom 39% suffered out-of-hospital CA; 87% had an initial non-shockable rhythm and the most frequent presumed cause was cardiac (31%). In-hospital mortality was 63%. Significant neurologic dysfunction (cerebral performance category 3 or 4) was seen in 31% of survivors at hospital discharge. Non-immediate initiation of basic life support (BLS), higher Simplified Acute Physiology Score II score and longer relative duration of vasopressor support were independent predictors of in-hospital mortality, while shockable rhythms were associated with improved survival. Higher Glasgow coma scale at ICU discharge and shorter length of ICU stay were predictors of better neurologic outcome.
ConclusionThis study highlights the positive prognostic impact of shockable rhythms, and confirms the importance of immediate initiation of BLS and prompt defibrillation, supporting the need for better training both outside and inside hospitals.
Doentes ressuscitados de uma paragem cardiorrespiratória (PCR) são admitidos em Unidades de Cuidados Intensivos (UCI) para receber cuidados pós-reanimação, mas apenas uma minoria sobrevive até à alta hospitalar. Compreender os fatores clínicos envolvidos na sua abordagem é essencial para melhorar o prognóstico.
ObjetivosCaracterizar a população admitida na UCI após reanimação bem-sucedida e analisar os fatores associados aos outcomes.
MétodosRealizamos um estudo retrospetivo e descritivo com doentes admitidos após PCR na UCI, de janeiro de 2014 a dezembro de 2018. Analisaram-se os fatores demográficos, caraterísticas da PCR, abordagem precoce e outcomes neurológico e de mortalidade.
ResultadosForam admitidos 187 doentes, com uma mediana de 67 anos; 39% sofreram PCR pré-hospitalar, 87% apresentavam ritmo inicial não desfibrilhável e a etiologia presumida mais frequente foi a cardíaca (31%). A mortalidade intra-hospitalar foi 63%; 31% dos sobreviventes tinha disfunção neurológica significativa à data da alta hospitalar (CPC 3 ou 4). O atraso no início do suporte básico de vida (SBV), score SAPS II mais elevado e maior duração indexada de suporte vasopressor foram preditores independentes de mortalidade intra-hospitalar. O ritmo desfibrilhável foi associado a melhoria da sobrevida. Um valor de Escala de Coma de Glasgow mais elevado na alta da UCI e menor duração de internamento na UCI foram preditores de melhor outcome neurológico.
ConclusãoEste estudo salienta o impacto prognóstico do ritmo desfibrilhável e confirma a importância do início imediato de SBV e da rápida desfibrilhação, reforçando a necessidade de capacitar a população intra e extra-hospitalar.
The majority of patients successfully resuscitated from cardiac arrest (CA) do not survive to hospital discharge, despite continuing advances in health care.1,2 This early mortality is generally caused by post-resuscitation circulatory failure (mainly due to systemic ischemia-reperfusion) and post-anoxic brain injury.3 In most countries, the presence of severe post-anoxic neurologic injury commonly results in a decision to withdraw life-sustaining treatment, accounting for half of deaths after CA.3 Intensive care units (ICUs) are frequently involved in the early management of these patients, to initiate the diagnostic workup and/or to provide post-resuscitation care.1,2
Survival rates after out-of-hospital cardiac arrest (OHCA) vary considerably worldwide and are highly dependent on the organization of emergency medical services (EMS), rates of bystander basic life support (BLS), time to first defibrillation, quality of advanced life support (ALS) and post-resuscitation care, i.e. the quality of the local chain of survival.4
On the other hand, most patients who have an in-hospital cardiac arrest (IHCA) will show signs of clinical deterioration in the hours preceding the event, and the probability of survival to hospital discharge decreases with increasing number and severity of organ dysfunctions.5 Recognizing those at risk of CA and timely initiation of appropriate therapeutic interventions is of the utmost importance for reducing the IHCA rate.4,6,7
When return of spontaneous circulation (ROSC) is achieved, determination and treatment of the cause of CA can prevent relapse and subsequent clinical deterioration.4 Functional outcomes of CA survivors are in part determined by their underlying health status and arrest-specific factors, but many aspects of medical care after CA may influence outcomes.8
It is essential to understand the clinical factors involved in the management of these patients in order to improve their prognosis. Therefore, this study aims to characterize patients admitted to the general ICU of a tertiary hospital after successful CA resuscitation, and to analyze factors associated with mortality and neurologic outcomes.
MethodsStudy settingWe performed a retrospective study including patients admitted within 24 hours of CA to the intensive care department (ICD) of Faro Hospital in southern Portugal, between 1 January 2014 and 31 December 2018. This department consists of a general ICU and a general intermediate care unit, served by a shared medical team. The ICD also provides a permanent in-hospital emergency program staffed by at least one doctor and one nurse, which responds promptly to in-hospital emergency situations (including CA) following direct telephone activation.
The ICU has a normothermia protocol to be implemented in CA patients who are comatose after ROSC, aiming for a target central (esophageal) temperature below 37°C (Supplementary Appendix). This protocol is contraindicated in some situations: patients with Glasgow coma scale (GCS) >10, severe hemodynamic instability, septic shock, cranial trauma, major bleeding or pregnancy. In accordance with the European Society of Intensive Care Medicine and the European Resuscitation Council,9,10 neurologic prognosis is assessed 72 hours after the index event, using the GCS and electroencephalogram (EEG), complemented by brain computed tomography (CT).
It should be noted that, in our hospital, patients who have suffered CA due to ST-elevation acute myocardial infarction or ventricular arrhythmias due to cardiological causes are generally admitted directly to the coronary intensive care unit in the cardiology department, and undergo emergent coronary angiography. Other patients are first admitted to the general ICU, and if the cause of CA is presumed to be myocardial infarction, in view of the patient's clinical course and if there is no other obvious cause, the case is discussed with the cardiology department and coronary angiography is performed whenever clinically indicated.
Withdrawal of life-sustaining treatment or ‘do not resuscitate’ decisions are determined after health care team discussion (including ICU doctors and nurses), according to neurologic prognosis and comorbidities. Some successfully resuscitated patients develop post-CA shock, which is defined as the need for continuous vasopressor support to maintain mean arterial pressure above 60 mmHg for more than six hours following ROSC, despite adequate fluid loading.3
Data collectionClinical information was extracted from electronic clinical records, using the clinical software SClinic® and on a running database using B-ICU.CARE®. An initial screening was performed by searching for the diagnosis “arrest”, applying the statistical functionality of B-ICU.CARE. All patients aged 17 years or more were included. Patients with respiratory arrest only, and those with cardiac arrest already admitted to the ICD for other causes, were excluded. Patients admitted 24 hours after CA were also excluded, mainly those transferred from the coronary intensive care unit or from other hospitals. Data were collected and organized following the Utstein Style guidelines11,12 whenever possible.
Demographic factors, CA characteristics, immediate and in-hospital approach (including electrocardiogram (ECG), brain CT, EEG, application of the normothermia protocol, vasopressors, inotropic agents and invasive mechanical ventilation [IMV]), CA recurrence, epileptic seizures, mortality and neurologic outcomes were analyzed. Myoclonus was included in the epileptic activity recorded since it is associated with a worse prognosis (especially if starting within 48 hours of ROSC), although some patients may survive with good outcome.10 Severity indices were recorded, based on the Simplified Acute Physiology Score (SAPS) II score and Sequential Organ Failure Assessment (SOFA) score in the first 24 hours and SOFA score at ICU discharge. Overall length of ICU stay was also reported for each patient. Regarding vasopressor support and IMV, the duration of support is presented in absolute values and also indexed to the total length of ICU stay, to minimize the effect of early mortality on the analysis. Our results are compared between subgroups of gender, location of arrest and clinical outcomes. Neurologic outcome was assessed by cerebral performance category (CPC) at hospital discharge and the best CPC and GCS recorded during ICU stay were reported. Survival outcome was assessed at hospital discharge, 28 days after discharge, at six months and at one year of follow-up.
Statistical analysisCategorical variables were described as absolute (n) and relative frequencies (%). Medians and percentiles were used for continuous variables. Mann-Whitney nonparametric tests were used as appropriate to test hypotheses concerning continuous variables, considering normality assumptions and the number of groups compared. The chi-square test and Fisher's exact test were used, as appropriate, to test hypotheses concerning categorical variables.
Univariate and multivariate logistic regression modeling was used to elucidate the factors associated with mortality and neurologic outcomes. The significance level used was 0.05. Statistical analysis was performed using IBM SPSS version 24.0.
ResultsCharacteristics of the study population and of cardiac arrestsDuring the five-year period, 187 patients were admitted to the ICD within 24 hours of successful cardiopulmonary resuscitation. Table 1 describes the documented study population and CA characteristics. The median age was 67 years and 121 patients (65%) were male; 61% of the CAs occurred in an in-patient setting and 80% were witnessed. However, bystander BLS was only performed in 73% of all patients. The first monitored rhythm was non-shockable in 87% of patients and the median time between CA and ROSC (downtime) was 10 min. Presumed cardiac causes accounted for 31% of CAs (acute coronary syndrome in 33% and acute or decompensated heart failure in 32%).
Characteristics of the study population and of cardiac arrests (n=187).
| Age, years, median (min-max) | 67 (17-92) |
| Gender, n (%) | |
| Male | 121 (65%) |
| Female | 66 (35%) |
| Location of arrest, n (%) | |
| Out-of-hospital | 73 (39%) |
| Home/hotel room | 25 (34%) |
| Other health care unit | 6 (8%) |
| Beach/swimming pool | 5 (7%) |
| Restaurant | 4 (6%) |
| Workplace | 2 (3%) |
| Car | 3 (4%) |
| Other public location | 11 (15%) |
| During transportation with EMS | 6 (8%) |
| Unknown | 11 (15%) |
| In-hospital | 114 (61%) |
| Ward | 41 (36%) |
| EMS | 25 (22%) |
| Emergency department | 20 (17%) |
| Operating room | 11 (10%) |
| Intermediate care unit | 9 (8%) |
| Other | 8 (7%) |
| Days until cardiac arrest in hospitalized patients, median (min-max) | 7 (0.5-81) |
| Time of cardiac arrest, n (%) | |
| Night (12 am-8 am) | 48 (26%) |
| Morning (8 am-4 pm) | 73 (39%) |
| Afternoon/evening (4 pm-12 am) | 66 (35%) |
| Median time | 1 pm |
| Peak time | 10 am |
| Witnessed arrest, n (%) | 149 (80%) |
| Bystander BLS, n (%) | 137 (73%) |
| First monitored rhythm, n (%) | |
| Non-shockable | 162 (87%) |
| Asystole | 79 (49%) |
| Pulseless electrical activity | 39 (24%) |
| Unspecified | 44 (27%) |
| Shockable | 24 (13%) |
| Pulseless ventricular tachycardia | 2 (8%) |
| Ventricular fibrillation | 16 (67%) |
| Unspecified | 6 (25%) |
| Downtime, min, median (min-max) | 10 (2-80) |
| GCS after ROSC (median) (P25-P75) | 3 (3-6) |
| Presumed cause of CA, n (%) | |
| Cardiac | 57 (31%) |
| Respiratory failure | 49 (26%) |
| Distributive shock | 17 (9%) |
| Metabolic or electrolyte disturbance | 15 (8%) |
| Hemorrhagic shock | 14 (7%) |
| Neurologic | 10 (5%) |
| Upper airway obstruction | 9 (5%) |
| Intoxication | 3 (2%) |
| Trauma | 3 (2%) |
| Multifactorial | 4 (2%) |
| Unknown | 6 (3%) |
| Presumed cardiac causes, n (%) | |
| Acute coronary syndrome | 19 (33%) |
| Acute or decompensated heart failure | 18 (32%) |
| Pulmonary embolism | 11 (19%) |
| Ventricular arrhythmia | 5 (9%) |
| Acute myocarditis | 4 (7%) |
| First ECG after ROSC, n (%) | |
| Sinus rhythm | 90 (48%) |
| Atrial fibrillation | 26 (14%) |
| Supraventricular tachycardia | 3 (2%) |
| Pacing rhythm | 3 (2%) |
| Junctional rhythm | 2 (1%) |
| Atrial flutter | 2 (1%) |
| Ventricular tachycardia with pulse | 2 (1%) |
| Not reported | 59 (31%) |
AV: atrioventricular; BLS: basic life support; ECG: electrocardiogram; EMS: emergency medical services; GCS: Glasgow Coma Scale; min: minimum; max: maximum; P25: 25th percentile; P75: 75th percentile; ROSC: return of spontaneous circulation.
Table 2 describes the in-hospital management of CA patients, 90% of whom were admitted to the ICU. The others rapidly recovered consciousness, no longer requiring IMV, and were admitted directly to the intermediate care unit. During hospitalization, the median duration of IMV was two days, and one day for vasopressor support (including both survivors and non-survivors). Norepinephrine was the main vasopressor drug used in these cases. The normothermia protocol was applied in 53% of patients, but 75 patients to whom it was not applied had some predefined contraindications, which gives a rate of 93% of application for eligible patients. Patients with suspected acute coronary syndrome or other cardiac cause that precipitated the CA (such as ventricular arrhythmia) were discussed with the cardiology department and transferred whenever this was considered to be the best strategy (10%). Nineteen percent of patients had epileptic activity and neurologic lesions were the main cause of post-CA dysfunction in 43%. Recurrence of CA with successful resuscitation (during transportation or in-hospital) occurred in 18%, with a maximum of six CAs in a single patient. The median length of ICU stay was three days, but it should be noted that extubated patients were transferred to the intermediate care unit to maintain monitored care. In-hospital mortality was 63% (118 patients), 45% of which was associated with withholding or withdrawal of life support. Fifty-five percent of the 69 patients who survived were classified as CPC 1.
In-hospital management and clinical course (n=187).
| Admission unit, n (%) | |
| Intensive care unit | 168 (90%) |
| Intermediate care unit | 19 (10%) |
| Severity indices, median (P25-P75) | |
| SAPS II score | 64 (54-76) |
| SOFA score in the first 24 hours | 11 (8-13) |
| SOFA score at ICU discharge | 9 (5-12.5) |
| Normothermia protocol, n (%) | |
| Initiated | 99 (55%) |
| Not initiated, due to: | 82 (45% |
| GCS >10 | 38 (46%) |
| Severe hemodynamic instability | 17 (21%) |
| Severe sepsis | 10 (12%) |
| Hemorrhagic shock | 8 (10%) |
| Severe head trauma | 1 (1%) |
| Pregnancy | 1 (1%) |
| Unknown | 7 (9%) |
| Invasive mechanical ventilation, median (P25-P75) | |
| Absolute duration, days | 2 (1-5) |
| Indexed to total length of ICU stay | 1 (0.5-1) |
| Vasopressor support, median (P25-P75) | |
| Absolute duration, days | 1 (0-2) |
| Indexed to total length of ICU stay | 0.35 (0-1) |
| Epileptic activity, n (%) | 35 (19%) |
| Myoclonus | 15 (43%) |
| Partial/focal seizure | 8 (23%) |
| Generalized tonic-clonic seizure | 7 (20%) |
| Nonconvulsive status epilepticus | 4 (11%) |
| Status epilepticus | 1 (3%) |
| Main dysfunction after CA, n (%) | |
| Neurologic | 81 (43%) |
| Shock | 65 (35%) |
| Multifactorial | 27 (14%) |
| None/unknown | 14 (7%) |
| Recurrence of CA, n (%) | 34 (18%) |
| Best CPC until ICU discharge, median (P25-P75) | 4 (2-4) |
| Best GCS until ICU discharge, median (P25-P75) | 8 (3-15) |
| GCS at ICU discharge (survivors only), median (P25-P75) | 15 (11-15) |
| Length of ICU stay, days, median (P25-P75) | 3 (1-8) |
| CPC score at hospital discharge, n (%) | |
| 1 | 38 (56%) |
| 2 | 9 (13%) |
| 3 | 13 (19%) |
| 4 | 8 (12%) |
CA: cardiac arrest; CPC: cerebral performance category; GCS: Glasgow Coma Scale; ICU: intensive care unit; P25: 25th percentile; P75: 75th percentile; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment.
Comparisons between the genders can be seen in Supplementary Table A. In our population, only SOFA score at admission reached a statistically significant difference, with higher median values in males (11 vs. 10; p=0.007). There were no statistically significant differences in mortality (62% vs. 65%, p=0.668) or neurologic outcomes (70% male patients with CPC 1-2 at discharge vs. 65% in females; p=0.203).
Comparison between locations of cardiac arrestSupplementary Table B shows comparisons between OHCA and IHCA. Statistically significant differences were observed in all variables except for gender (p=0.810), downtime (p=0.069), duration of IMV (p=0.132) and of vasopressor support (p=0.295), epileptic activity (p=0.200), GCS at ICU discharge (p=0.874) and neurologic outcome (p=0.887). There was also no statistically significant difference in mortality (63% in both groups, p=0.984).
Regarding OHCA, a smaller proportion of these patients had immediate initiation of BLS (52% vs. 87%, p<0.001), with a lower GCS after ROSC (3.8 vs. 5.0; p=0.003), while the ward was the main location of arrest in IHCA (22%). These patients less often had shockable rhythms at initial assessment (6% vs. 25%, p=0.001), and hence less presumed cardiac causes of CA (22% vs. 44%, p=0.002). Regarding the clinical conditions that led to hospital admission, they also had higher severity indices at ICU admission (p<0.001) and shock was the main dysfunction after CA (40% vs. 23%, p=0.016).
Mortality analysisMortality is analyzed in Table 3. Of the 118 non-surviving patients (63%), 64% were male and 61% suffered IHCA. The normothermia protocol was applied in 87% of patients without contraindications, and 19% experienced recurrence of CA with successful resuscitation. Nineteen percent presented epileptic activity. Patients who died during hospitalization less often had bystander BLS (68% vs. 83%, p=0.027), and had more frequent non-shockable rhythm at initial assessment (93% vs. 77%, p=0.001) and longer median downtime (10 vs. 8 min; p=0.052). All severity indices were higher (p<0.001) and these patients had longer indexed durations of IMV (1.0 vs. 0.50; p<0.001) and vasopressor support (0.6 vs. 0.3; p<0.001) indexed to total length of ICU stay. Survivors had higher GCS after ROSC (5 vs. 3; p=0.003) and longer median hospital length of stay (five vs. two days; p<0.001).
Mortality analysis.
| Survivors (n=69) | Non-survivors (n=118) | p | |
|---|---|---|---|
| Characteristics of CA | |||
| Age, years, median (P25-P75) | 64 (54-75) | 68 (57-81) | 0.08b |
| Gender, n (%) | 0.668a | ||
| Male | 46 (67%) | 75 (64%) | |
| Female | 23 (33%) | 43 (36%) | |
| Location of arrest, n (%) | 0.984a | ||
| Out-of-hospital | 27 (39%) | 46 (39%) | |
| In-hospital | 42 (61%) | 72 (61%) | |
| Witnessed arrest, n (%) | 61 (88%) | 88 (74%) | 0.023a |
| Initiation of BLS, n (%) | 57 (83%) | 80 (68%) | 0.027a |
| First monitored rhythm, n (%) | 0.001a | ||
| Non-shockable | 53 (77%) | 109 (93%) | |
| Shockable | 16 (23%) | 8 (7%) | |
| Downtime, min, median (P25-P75) | 8 (6-15) | 10 (6-20) | 0.069b |
| GCS after ROSC, median (P25-P75) | 5 (3-6) | 3 (3-4) | 0.003b |
| Presumed cause of CA, n (%) | |||
| Cardiac | 25 (36%) | 32 (27%) | 0.191a |
| Respiratory failure | 18 (26%) | 31 (26%) | |
| Management in ICU | |||
| Severity indices, median (P25-P75) | |||
| SAPS II score | 55 (47-64) | 70 (62-82) | <0.001b |
| SOFA score in the first 24 hours | 10 (8-11) | 12 (10-14) | <0.001b |
| SOFA score at ICU discharge | 4 (2-6) | 12 (9-14) | <0.001b |
| Normothermia protocol in patients without contraindications, n (%) | 32 (91%) | 67 (87%) | 0.499a |
| IMV, median (P25-P75) | |||
| Absolute duration, days | 3.0 (0.5-6) | 1.5 (0.5-4) | 0.144b |
| Indexed to total length of ICU stay | 0.5 (0.29-0.75) | 1.0 (1-1) | <0.001b |
| Vasopressor support, median (P25-P75) | |||
| Absolute duration, days | 1.0 (0-3) | 1.0 (0-2) | 0.295b |
| Indexed to total length of ICU stay | 0.17 (0-0.5) | 1.0 (0-1) | <0.001b |
| Post-CA shock, n (%) | 18 (26%) | 45 (38%) | 0.093a |
| Epileptic activity, n (%) | 13 (19%) | 22 (19%) | 0.973a |
| Best GCS, median (P25-P75) | 15 (13.5-15) | 4 (3-9) | <0.001b |
| Best CPC, median (P25-P75) | 1 (1-3) | 4 (4-4) | <0.001b |
| Recurrence, n (%) | 12 (17%) | 22 (19%) | 0.325a |
| Length of ICU stay, days, median (P25-P75) | 5 (2-12) | 2 (1-6) | <0.001b |
BLS: basic life support; CA: cardiac arrest; CPC: cerebral performance category; GCS: Glasgow Coma Scale; ICU: intensive care unit; IMV: invasive mechanical ventilation; P25: 25th percentile; P75: 75th percentile; ROSC: return of spontaneous circulation; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment.
After multivariate analysis, non-immediate initiation of BLS (odds ratio [OR] 6.040 [1.533-23.801], p=0.010), higher SAPS II score (OR 1.105 [1.048-1.165], p<0.001) and higher indexed duration of vasopressor support (OR 8.375 [1.722-40.726], p=0.008) were independent predictors of in-hospital mortality. Shockable rhythms (OR 0.129 [0.024-0.791], p=0.018) were independently associated with improved survival.
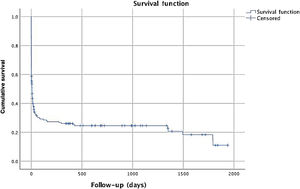
Median follow-up was seven days, with a mean of 235 days, since all patients were included and most of them died in-hospital. Mortality at 28 days and 12 months after discharge was 67% and 72%, respectively. Figure 1 reveals cumulative survival during follow-up, indicating higher mortality within six months of CA. Comparison of cumulative survival according to first monitored rhythm and initiation of BLS is shown in Figure 2.
Analysis of neurologic outcomesOf the 69 survivors, 31% had significant neurologic dysfunction (CPC 3 or 4) at discharge. Comparing CPC 1 or 2 patients with CPC 3 or 4 patients (Table 4), statistically significant differences were found concerning IMV duration (two vs. eight days, p<0.001), GCS (15 vs. 10, p<0.001), SOFA score (3.5 vs. 5, p=0.008) and length of ICU stay (four vs. 12 days, p<0.001). Also, CPC 3 or 4 patients had a higher incidence of epileptic activity (38% vs. 9%, p=0.015) and lower rates of post-CA shock (95% vs. 34%, p=0.003).
Neurological outcomes.
| CPC 1 or 2 (n=47) | CPC 3 or 4 (n=21) | p | |
|---|---|---|---|
| Characteristics of CA | |||
| Age, years, median (P25-P75) | 64 (52-75) | 70 (57.5-75) | 0.528b |
| Gender, n (%) | 0.619a | ||
| Male | 32 (68%) | 13 (62%) | |
| Female | 15 (32%) | 8 (38%) | |
| Location of arrest, n (%) | 0.578a | ||
| Out-of-hospital | 19 (40%) | 7 (33%) | |
| In-hospital | 28 (60%) | 14 (67%) | |
| Time to CA in hospitalized patients, days, median (P25-P75) | 4 (2-8) | 12 (9-17) | 0.057b |
| Witnessed arrest, n (%) | 40 (85%) | 20 (95%) | 0.231a |
| Initiation of BLS, n (%) | 38 (81%) | 19 (91%) | 0.319a |
| First monitored rhythm, n (% | 0.560a | ||
| Non-shockable | 35 (75%) | 17 (81%) | |
| Shockable | 12 (25%) | 4 (19%) | |
| Downtime, min, median (P25-P75) | 8 (6-15) | 6 (4-6) | 0.704b |
| GCS after ROSC, median (P25-P75) | 5 (3-7) | 3.5 (3-6) | 0.339b |
| Presumed cause of CA, n (%) | 0.349a | ||
| Cardiac | 19 (40%) | 6 (29%) | |
| Respiratory failure | 14 (30%) | 4 (19%) | |
| Management in ICU | |||
| Severity indices, median (P25-P75) | |||
| SAPS II score | 52 (43.75-61) | 58 (51-66.5) | 0.126b |
| SOFA score in the first 24 hours | 10 (7-11) | 9 (7.5 -11) | 0.800b |
| SOFA score at ICU discharge | 3.5 (1-6) | 5 (4-9) | 0.008b |
| Normothermia protocol, n (%) | 20 (44%) | 11 (55%) | 0.389a |
| Invasive mechanical ventilation, median (P25-P75) | |||
| Absolute duration, days | 2 (0.5-3) | 8 (3.5-17.5) | <0.001b |
| Indexed to total length of ICU stay | 0.5 (0.25-0.67) | 0.8 (0.67-1) | <0.001b |
| Vasopressor support, median (P25-P75) | |||
| Absolute duration, days | 1 (0-3) | 2 (0-4) | 0.404b |
| Indexed to total length of ICU stay | 0.25 (0-0.5) | 0.10 (0-0.5) | 0.843b |
| Post-CA shock, n (%) | 16 (34%) | 2 (9.5%) | 0.003a |
| Epileptic activity, n (%) | 4 (9%) | 8 (38%) | 0.015a |
| Best GCS, median (P25-P75) | 15 (15-15) | 10 (7-14.5) | <0.001b |
| Best CPC, median (P25-P75) | 1 (1-1) | 3 (3-4) | <0.001b |
| GCS at ICU discharge, median (P25-P75) | 15 (15-15) | 10 (4.5-13) | <0.001b |
| Length of ICU stay in days, median (P25-P75) | 4 (2-7) | 12 (5-20.5) | <0.001b |
BLS: basic life support; CA: cardiac arrest; CPC: cerebral performance category; GCS: Glasgow Coma Scale; ICU: intensive care unit; IMV: invasive mechanical ventilation; P25: 25th percentile; P75: 75th percentile; ROSC: return of spontaneous circulation; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment.
On multivariate regression analysis using GCS at ICU discharge, length of ICU stay, and SOFA at ICU discharge as independent variables, GCS (OR=0.727 [0.569-0.927]) and length of ICU stay (OR 1.153 [1.027-1.295]) remained statistically significantly associated with CPC. Although post-CA shock was associated with a better outcome in univariate analysis, multivariate analysis showed that, after adjusting for epileptic activity and IMV, it was no longer statistically significantly associated with CPC (OR=3.843 [0.700-21.100], p=0.121).
DiscussionThe present study has five main findings. First, non-immediate initiation of BLS, higher SAPS II score and longer indexed duration of vasopressor support were found to be independent predictors of in-hospital mortality, while shockable rhythms were associated with improved survival. In-hospital and one-year mortality rates were high, but lower than in previous reports.13–15 One explanation for this phenomenon could be the organization of the EMS, particularly in-hospital: in those patients who survived, BLS was more frequently initiated and GCS after ROSC and the prevalence of shockable rhythms were higher. Shockable rhythms can be treated successfully with defibrillation, but prompt initiation of BLS is also crucial, and is in fact the foundation of the chain of survival.1
Second, patients discharged in CPC 1 or 2 had significantly less frequent epileptic activity and a shorter course of ventilatory support. Sixty-eight percent of survivors were discharged in CPC 1 or 2, but the others had significant neurologic impairment. We highlight the longer hospitalization prior to IHCA in patients discharged in CPC 3 or 4, which, although not reaching statistical significance, points to the need for closer monitoring in hospitalized patients in order to detect early warning signs of clinical deterioration.4–7 IHCA patients with prolonged hospitalization also have an increased prevalence of comorbidities and are usually in worse clinical condition, resulting in a poor prognosis.
Third, regardless of the disparities in the immediate management of OHCA and IHCA, there were no statistically significant differences between clinical outcomes in our population based on the location of arrest, a finding that has not been previously published. Notwithstanding, immediate BLS and downtime in patients who suffered OHCA were worse. Longer resuscitation is associated with tissue hypoperfusion and hypoxic damage,1 which explains the lower GCS after ROSC in this subgroup and the more frequent implementation of the normothermia protocol. The in-hospital EMS are central to ensuring lower downtimes, since appropriate activation leads to prompt and organized intervention of specialized support, increasing the odds of survival.16
Fourth, there were no significant differences between the genders in mortality or neurologic outcomes, unlike some studies which report worse survival in women,17,18 possibly due to the smaller number of females. Aside from admission SOFA score, which was higher in men, no other comparison between the genders revealed statistically significant differences.
Fifth, post-resuscitation brain injury was the main cause of death, leading to neurologic withdrawal of care in some patients. Although the overall median duration of IMV was two days, indexed IMV duration was longer in non-survivors, and patients with worse neurologic evolution also needed longer courses, partly to enable correct neuroprognostication after CA. Better neurologic outcome was significantly associated with higher GCS at ICU discharge and shorter length of ICU stay. Neither epileptic activity nor IMV reached statistical significance. To our knowledge, these findings have not been previously reported.
Several limitations of our study should be noted. It is retrospective in nature and all data were extracted from a running database and electronic clinical records, which may be of variable accuracy. Also, not all the clinical records were in accordance with the Utstein Style guidelines, so some information was incomplete. This missing data could have impacted the statistical significance of the results. Finally, our study does not include patients admitted directly to the coronary intensive care unit with ST-elevation myocardial infarction or other cardiac cause for the CA identified before hospital admission. Consequently, the prevalence of cardiac causes (and the percentage of shockable rhythms) is lower than in other series, which affects the clinical outcomes. However, while also including cardiac causes of CA, our study provides a better understanding of multiple other causes that are generally less explored and with a poorer prognosis.
ConclusionMortality after CA remains high despite advances in ALS and post-resuscitation care. It is critical to understand the factors associated with better survival and neurologic outcome, in order to improve management in post-CA patients. Shockable rhythms were associated with improved survival, while non-immediate initiation of BLS, higher SAPS II score and longer indexed duration of vasopressor support were independent predictors of in-hospital mortality.
This study confirms the importance of the pre-hospital approach, including immediate initiation of BLS and prompt defibrillation, supporting the need for better training both outside and inside hospitals. Timely high-quality BLS, early defibrillation, and optimal post-resuscitation care significantly impact clinical outcome.
Ethics statementThis study was approved by the Ethics Committee of Centro Hospitalar Universitário do Algarve (Faro Hospital).
FundingNot applicable.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to thank the Municipal Council of Loulé for all the support they provided during the study.