Superior vena cava (SVC) syndrome is typically dramatic. Although the diagnosis is usually easy, elucidation of the etiology is difficult. We present a patient who developed SVC syndrome early after transvenous pacemaker implantation and who was subsequently diagnosed with lung carcinoma. The pathogenesis seems to be explained by a combination of two etiologies: lung carcinoma plus transvenous pacemaker implantation. We emphasize that common etiologies such as malignancy should be considered first when faced with SVC syndrome.
A síndrome da veia cava superior (VCS) é normalmente uma situação dramática. Embora o diagnóstico seja habitualmente fácil, todo o esclarecimento sobre esta etiologia é difícil. Apresentamos o caso de um doente que desenvolveu a síndrome da VCS logo após a implantação de um pacemaker em que o diagnóstico de carcinoma no pulmão foi estabelecido posteriormente. A patogénese parece ser explicada por uma combinação de duas etiologias patogénicas: o carcinoma do pulmão e a implantação de um pacemaker transvenoso. Destacamos que as etiologias comuns tais como a malignidade devem ser consideradas em primeiro lugar ao enfrentar a síndrome da VCS.
Superior vena cava (SVC) syndrome, first described by William Hunter in 1757, is typically dramatic. Although the diagnosis is usually easy, elucidation of the etiology is more difficult. In the past, infectious lesions such as syphilitic aortic aneurysm were common causes, but nowadays malignancy such as bronchial carcinoma or mediastinal lymphoma, and the use of intravascular devices and cardiac pacemakers, have become the main causes.1,2
In this paper, we report an unusual case that presented with SVC syndrome early after pacemaker implantation and diagnosis of lung carcinoma.
Case reportThe patient was an 84-year-old male with a medical history of type 2 diabetes and symptomatic sick sinus syndrome. He was treated by permanent transvenous single-chamber pacing (VVI) via the right subclavian vein (Talos SR, Biotronik) the day after admission to our hospital.
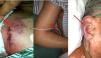
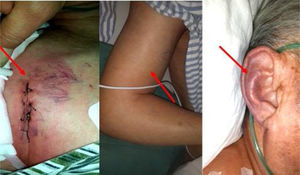
On the second postoperative day, the patient developed cyanotic lips and ears, and swelling of the face, neck, and bilateral upper extremities. These symptoms worsened gradually. Physical examination revealed raised jugular venous pressure and distended superficial veins of the chest at the site of the pacemaker implantation, consistent with SVC syndrome (Figure 1).
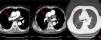
The patient had elevated D-dimers (6.93 mg/l, normal 0-0.55 mg/l) and two tumor markers (Ca19-9 59.45 U/ml, normal 0-37 U/ml; Ca-211 4.24 ng/ml, normal 0-3.3 ng/ml). He also presented with hypoxemia (pH: 7.48, pO2: 7.30 kPa, pCO2: 3.70 kPa, SO2: 91.00%) in arterial blood gas analysis. He underwent a duplex venous scan of the upper extremities and neck, which showed extensive thrombosis of bilateral axillary veins. A computed tomography (CT) chest scan with intravenous contrast showed thrombosis of the SVC with multiple collateral vessels and alterations indicative of chronic bronchitis. However, it was not clear from the CT whether there was extrinsic compression on the SVC due to interference from the pacemaker electrode.
The patient was prescribed anticoagulation with enoxaparin and warfarin, and traditional Chinese medicine (including hirudo, lumbricus, ginseng and scorpion venom) by intravenous drip and oral administration, in order to promote blood circulation and prevent blood stasis. After one month of therapy, symptoms began to improve, with decreased edema of the face, neck and arms and disappearance of the superficial veins of the chest. The duplex venous scan showed no evidence of thrombosis of bilateral axillary veins.
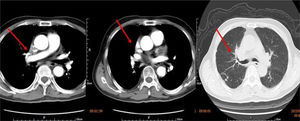
We retrieved the patient's CT scan performed three months previous to this hospitalization for chronic dry cough and shortness of breath, and discovered that tissue derived from the upper right mediastinum (the source probably being pulmonary tissue) had already severely compressed and narrowed the SVC. Obstructive pneumonia, a pulmonary metastatic node and bronchial stenosis could also be seen on this CT (Figure 2). However, a diagnosis had been missed on the basis of the previous CT scan, which had not been paid due attention. So finally lung carcinoma was considered for this patient, owing to the presence of SVC obstruction, the signs of the CT scan performed three months previously, elevated tumor markers and history of chronic dry cough. The patient refused any invasive intervention for examination or therapy, but was willing to undergo long-term anticoagulation (warfarin) and cancer therapy with traditional Chinese medicine. No recurrence of SVC syndrome was seen at one month of follow-up, but sadly, two months after discharge he died from traumatic subarachnoid hemorrhage related to anticoagulant therapy.
DiscussionSVC syndrome results from the obstruction of blood flow through the SVC into the right atrium. Generally, malignancy is considered to be the most common etiology of SVC syndrome, but benign iatrogenic causes, mainly intravascular devices (catheters, cardiac defibrillators and pacemaker wires), are becoming increasingly common.1 Obstruction can be caused by invasion or external compression of the SVC by adjacent pathologic processes involving the lung, lymph nodes, and other mediastinal structures or by thrombosis of blood within the SVC.2 Malignancy may not only narrow the SVC by invasion or external compression but also by changes in the coagulation and fibrinolytic systems, leading to a hypercoagulable state. SVC thrombosis with or without stenosis from pacemaker leads was described by Kosowsky and Barr in 1972. SVC syndrome is an extremely rare but serious complication after pacemaker lead implantation, characterized by symptomatic occlusion of the SVC.3 Most patients with SVC syndrome are asymptomatic due to the development of an adequate venous collateral circulation. Symptomatic cases of SVC syndrome from transvenous pacemaker implantation are rare, thought to be less than 0.5% of all patients.4 The mechanism may be mechanical stress caused by the transvenous leads, causing inflammation of the blood vessel wall and fibrosis, eventually leading to venous thrombosis and occlusion. In the case presented, lung carcinoma, by causing external compression of the SVC and a hypercoagulable state, may explain the SVC obstruction. However, the use of venous catheters may have enhanced venous thrombosis as a result of active inflammation in the endothelium. Balloon angioplasty or surgery was considered, as in other reports, but the patient refused. If the diagnosis of lung carcinoma has been recognized before the indication for a pacemaker, an epicardial route might have been preferred for pacemaker implantation.5
ConclusionSVC syndrome is usually easily diagnosed but elucidation of the etiology is more difficult. In this case report we present a patient who developed SVC syndrome early after transvenous permanent pacemaker implantation and who was subsequently diagnosed with lung carcinoma. The pathogenesis seems to be explained by a combination of two etiologies: lung carcinoma plus transvenous pacemaker implantation. Although nowadays the extensive use of intravascular devices and cardiac pacemakers has become one of the most common causes of SVC syndrome, common etiologies such as malignancy should be considered first when faced with this complicated disease.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by a college project of Putuo Hospital, Shanghai University of Traditional Chinese Medicine (2016316A) to Liting Pan, National Natural Science Foundation of China (81370331) to Daying Wang, Shanghai medical key specialty construction plan – cardiology (ZK2015A17).