Spontaneous coronary artery dissection (SCAD) represents 1–4% of all acute coronary syndromes (ACS), and is a particularly important cause among young women and individuals with few cardiovascular risk factors.
ObjectivesTo characterize clinical background, therapeutic management and clinical outcomes in a SCAD population.
MethodsWe retrospectively analyzed all consecutive patients diagnosed with SCAD at a tertiary center between August 2009 and May 2020, with a median follow-up of 40 months (IQR 14–95 months). SCAD was classified according to the Saw angiographic SCAD classification.
ResultsA total of 36 patients were included, 94% female, mean age 51 years (±11 years). A trigger was only detected in 8% and associated conditions in 31% of patients, mainly inflammatory or autoimmune systemic diseases and migraine. Most patients had non-ST-elevation ACS and 33% presented with ST-elevation ACS. The most frequent culprit lesion was the left anterior descending (LAD) artery (67%); mid to distal segments were the most affected (94%) and type 2 dissection the most prevalent (60%). Almost all patients were successfully medically managed, with only four undergoing percutaneous intervention. During follow-up, ischemic events recurred in 15% of patients and no patient died. Patients with type 2 dissection exhibited lower risk of recurrence compared to type 1 (p=0.049, OR=0.13).
ConclusionSCAD patients were mainly young or middle-aged women; the LAD artery was the most affected vessel and type 2 dissection the most prevalent. This report showed for the first time a correlation between type 2 SCAD and lower risk of recurrence.
A disseção coronária espontânea (DCE) representa 1 a 4% de todas as síndromes coronárias agudas (SCA), sendo uma causa particularmente importante em jovens mulheres e indivíduos com poucos fatores de risco cardiovasculares.
ObjetivosCaracterização de uma população diagnosticada com DCE, abordagem terapêutica e evolução clínica a longo prazo.
MétodosAnálise retrospetiva de todos os doentes consecutivamente diagnosticados com DCE num centro terciário, entre agosto de 2009 e maio de 2020, com um seguimento médio de 40 meses (IQR 14-95 meses). A DCE foi classificada de acordo com a classificação angiográfica de Saw.
ResultadosForam incluídos 36 doentes, 94% mulheres com média de 51 anos (±11-anos). Apenas 8% apresentavam um trigger e 31% apresentavam uma condição associada, especialmente doença inflamatória ou autoimune sistémica ou história de enxaqueca. A maioria teve um SCA sem supradesnivelamento-do-segmento-ST e 33% uma SCA com supradesnivelamento-de-ST. A artéria responsável mais comum foi a descendente anterior (67%); os segmentos médios e distais foram os mais afetados (94%) e a disseção tipo 2 foi a mais prevalente (60%). A maioria foi tratada medicamente com sucesso, sendo que apenas quatro doentes foram submetidos a angioplastia. Durante o seguimento, houve recorrência de eventos em 15% dos doentes e nenhum faleceu. A disseção tipo 2 correlacionou-se significativamente com menor risco de recorrência face à disseção tipo 1 (p=0,049, OR=O,13).
ConclusãoA maioria dos doentes era mulher jovem ou de meia-idade; a artéria descendente anterior foi a mais afetada e a disseção tipo 2 a mais prevalente. Este trabalho mostrou pela primeira vez uma correlação entre a DCE tipo 2 e menor risco de recorrência de eventos.
Spontaneous coronary artery dissection (SCAD) is defined as a non-atherosclerotic and non-traumatic separation of the layers of an epicardial coronary artery wall by intramural hemorrhage, which creates a false lumen with intramural hematoma that compresses the true lumen, causing myocardial ischemia.1,2 Classically, SCAD was considered a rare cause of acute coronary syndrome (ACS). However, it has emerged as an important cause of myocardial infarction (MI) among young people, accounting for up to 35% of ACS in women under 50 years old and up to 4% of all ACS. Nevertheless, since it is often misdiagnosed or underdiagnosed, its true incidence remains unknown.1–7 Coronary angiography remains the gold standard for diagnosis, while intracoronary imaging with optical coherence tomography (OCT) or intravascular ultrasound (IVUS) can make it easier to recognize in unclear situations.1,3 Saw classified three types of SCAD according to their distinct angiographic appearance: type 1 refers to the classical appearance with contrast dye staining of the arterial wall with multiple radiolucent lumen; type 2 is characterized by diffuse stenosis (typically of more than 20 mm) with abrupt change in arterial caliber and variable severity; and type 3 mimics atherosclerosis, with relatively linear stenosis.8 Once the diagnosis is made, medical treatment is implemented successfully in the majority of patients,2 but evidence-based guidance for optimal acute and long-term care is lacking.7
MethodsWe retrospectively analyzed all consecutive patients diagnosed with SCAD between August 2009 and May 2020 at Centro Hospitalar Universitario São João, a tertiary center in Porto, Portugal. Patients with iatrogenic or atherosclerotic plaque dissection were excluded. Clinical data were collected, and coronary angiography records were reviewed by investigators and by two cardiac intervention experts to ensure appropriate definition. The Saw angiographic classification of SCAD3 was applied and the American Heart Association coronary artery disease reporting system was used to define the affected coronary segment.9,10
A total of 36 patients were identified and enrolled in the study. Clinical, imaging, and angiographic data were collected at admission and during a median follow-up of 40 months (interquartile range [IQR] 14–95 months), until April 2021. No patients were lost to follow-up.
Pain, events, SCAD recurrence, and death from any cause were analyzed. SCAD recurrence was defined as a new ST-elevation myocardial infarction (STEMI), non-STEMI (NSTEMI) or unstable angina, not related to extension of the original SCAD dissection. Angiographic healing was defined as improvement in stenosis severity from the index event, residual stenosis <50% and Thrombolysis in Myocardial Infarction (TIMI) flow grade 3.11
Data are presented as mean±standard deviation (SD) for paired continuous variables or median (IQR) for unpaired continuous variables, and as number and percentage for categorical variables. The statistical analysis was performed in IBM SPSS Statistics version 25. The one-sample Kolmogorov-Smirnov test was performed to assess normality of distribution. Categorical variables were compared using the chi-square test. Differences were considered statistically significant with a p-value ≤0.05. The Kaplan-Meier method was used to estimate survival curves for follow-up events.
The study was approved by the institutional ethics committee.
ResultsOf a total of 36 patients with SCAD, 94% (n=34) were female, mean age was 51±11 years, and 36% (n=13) presented no cardiovascular risk factors. The population's baseline characteristics are summarized in Table 1. Seventeen (47%) women were postmenopausal, with only one on hormone replacement therapy. Of the 17 women of childbearing age (47%), eight were on hormonal contraception, mostly oral. Only three patients (8%) had an obvious trigger for SCAD: a male who did vigorous exercise, a female subjected to emotional stress and another female with recent delivery (one week before). Associated conditions were prevalent among the sample: 8% had inflammatory systemic disease, 8% (n=3) had autoimmune disease, 8% (n=3) suffered from migraine, 6% (n=2) were on hormonal therapy and 3% (n=1) were pregnant. Also, 22% (n=8) of patients were previously diagnosed with depression, 11% (n=4) had anxiety disorder, 3% (n=1) had irritable bowel syndrome and 3% (n=1) had fibromyalgia (similar prevalences to the general population). Regarding the acute event, most patients (61%, n=22) had NSTEMI, 33% (n=12) had STEMI and 6% (n=2) had unstable angina. Most patients were in Killip class I (94%, n=34) and had normal left ventricular systolic function (72%, n=26).
Baseline characteristics of patients admitted with spontaneous coronary artery dissection (n=36).
| Age, years, mean±SD | 51±11 |
| Female, n (%) | 34 (94) |
| Hypertension, n (%) | 15 (53) |
| Dyslipidemia, n (%) | 14 (39) |
| Diabetes, n (%) | 2 (6) |
| Smoker or previous smoker, n (%) | 11 (31) |
| Family history of coronary artery disease, n (%) | 3 (8) |
| Acute event, n (%) | |
| STEMI | 12 (33) |
| NSTEMI | 22 (61) |
| Unstable angina | 2 (6) |
| Killip class, n (%) | |
| I | 30 (94) |
| II | 2 (6) |
| Left ventricular systolic function, n (%) | |
| Normal | 26 (72) |
| Mildly reduced | 5 (14) |
| Moderately reduced | 5 (14) |
| Median troponin, ng/l (IQR) | 6650 (3532–18294) |
IQR: interquartile range; NSTEMI: non-ST-elevation myocardial infarction; SD: standard deviation; STEMI: ST-elevation myocardial infarction.
All patients underwent coronary angiography (Table 2), by a femoral or a radial approach (52.9% and 47.1%, respectively). About 14% of patients presented complications: two had a local radial access complication and two had dissection propagation during the intervention.
Coronary angiography characteristics.
| Coronary territory affected, n (%) | |
| LAD | 24 (67) |
| LCx | 9 (25) |
| RCA | 6 (17) |
| LM | 2 (6) |
| Multivessel SCAD, n (%) | 4 (11%) |
| SCAD type, n (%) | |
| 1 | 14 (40) |
| 2 | 21 (60) |
| 3 | 0 (0) |
| TIMI flow grade, n (%) | |
| 0 | 8 (23.5) |
| 1 | 6 (17.6) |
| 2 | 5 (14.7) |
| 3 | 15 (44.1) |
| Stenosis severity, %, median (IQR) | 90 (77–100) |
| Access route, n (%) | |
| Radial | 18 (52.9) |
| Femoral | 16 (47.1) |
IQR: interquartile range; LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main coronary artery; RCA: right coronary artery; SCAD: spontaneous coronary artery dissection; TIMI: Thrombolysis in Myocardial Infarction.
The coronary territory most frequently affected was that of the left anterior descending (LAD) artery, in 67% (n=24) of patients, followed by the left circumflex in 25% (n=9), right coronary artery in 17% (n=6) and left main (LM) coronary artery in 6% (n=2). Only four patients (11%) presented multivessel dissection, two involving the LM. Mid to distal segments were the most affected (94%, n=34), particularly the distal LAD (41.7%) and obtuse marginal (16.7%). Type 2 dissection was the most prevalent (60%, n=21); no type 3 dissection was diagnosed. Percutaneous intervention with balloon and stent implantation were performed in one and three patients, respectively. These patients had TIMI flow grade 1 or 0; one of them had LM dissection with hemodynamic instability. The other patient with LM dissection was successfully managed medically.
Overall, 26% (n=9) of patients had pain recurrence and 37% (n=13) underwent repeat coronary angiography during hospitalization. Even with disease progression in 53% (n=8) of patients, no intervention was performed.
Concerning diagnostic workup, 11% (n=4) of patients underwent computed tomography (CT) angiography and 11% (n=4) renal artery Doppler; all exams were normal. Additionally, immunological tests were performed in 43% (n=18) of patients, of which 17% (n=3) were abnormal.
At discharge, 92% (n=33) of patients were prescribed aspirin (83% [n=30] dual anti-platelet therapy, mostly clopidogrel), 8% (n=3) anticoagulants, 72% (n=26) statins, 78% (n=28) beta-blockers and 61% (n=22) renin-angiotensin system inhibitors (RASi).
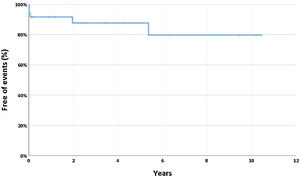
During a median follow-up of 40 months (IQR 14–95 months), most patients (67%, n=24) underwent a functional coronary stress test, with only two presenting significant ischemia. Seven patients (19%) underwent repeat coronary angiography (after a median of six months) due to pain recurrence, ischemia or operator decision; all patients presented improvement in the dissection (half with angiographic healing). During follow-up, chest pain occurred in 19% (n=7) and 14% (n=5) had SCAD recurrence, all non-ST-elevation ACS, after a median of 21 days (IQR 6-1340 days). Figure 1 displays time free of SCAD recurrence during follow-up. No association was found between the use of beta-blockers, RASi, statins or percutaneous interventions and SCAD recurrence during follow-up (p=0.898, p=0.297, p=0.511 and p=0.496, respectively). Patients with type 2 dissection exhibited lower risk of recurrence (p=0.049, OR=O.13 [95% confidence interval 0.01-1.27]). No patients died during follow-up.
Recurrence of spontaneous coronary artery dissection (SCAD) during follow-up. The Kaplan-Meier curve shows time free of recurrence, which was defined as a new ST-elevation myocardial infarction, non-ST-elevation myocardial infarction or unstable angina, and which did not involve extension of dissection of the original SCAD.
Our report, in agreement with studies that identify the typical SCAD patient as a middle-aged woman with few cardiovascular risk factors,7 showed that almost all patients with SCAD were women, with a mean age of 51 years. This raises questions about the true incidence and prevalence of the disease: young patients, and particularly young females, may be under-referred for coronary disease assessment or misdiagnosed due to the perception that coronary artery disease should not affect young and otherwise healthy people.12
The pathogenesis of SCAD is poorly understood, but appears to be heterogeneous, including possible inherited or acquired arteriopathies, prothrombotic states, predisposing genetic factors, hormonal influences, or systemic inflammatory diseases.3,13,14 Although studies have not shown a long-term benefit of extra-coronary vascular imaging after SCAD, the prevalence of coexisting arterial abnormalities outside the heart and specifically the association between SCAD and fibromuscular dysplasia (FMD), documented in 41–86% of patients,15 explains why major consensus documents recommend arterial imaging from head to pelvis, usually with CT or magnetic resonance angiography.2,7 In our study, 22% of patients underwent additional non-cardiac imaging that proved to be negative, precluding conclusions about the prevalence of FMD in our population.
With regard to other associated diseases, one third of patients had a concomitant condition, mostly inflammatory or autoimmune disease, or migraine. This is in agreement with previous reports, such as a Canadian study by Saw et al. in which 8.9% of patients presented systemic inflammatory conditions.14 Furthermore, results from a population-based cohort study in the USA consistently showed that SCAD was associated with migraines and some autoimmune and inflammatory conditions.13
Most patients presented with a non-ST-elevation ACS and were in Killip class I. As described in the literature, the LAD and mid-distal segments were the most affected and type 2 dissection was the most prevalent. Since routine IVUS or OCT were not performed, type 3 SCADs may have been misdiagnosed.
Concerning treatment, a conservative approach was adopted in most patients, with only 11% undergoing percutaneous intervention (PCI). There have been no randomized control trials comparing medical therapy to revascularization strategies in SCAD, however neither PCI nor coronary artery bypass grafting (CABG) seem ideal.1,2 In PCI there is a risk of iatrogenic injury or stent malposition as a result of intramural hematoma reabsorption, while in CABG, long-term patency of the bypass graft is poor due to recanalization of the native coronary.2 Evidence shows that most cases of SCAD stabilize and heal completely over time if managed conservatively,15 and the European Society of Cardiology and American Heart Association/American College of Cardiology guidelines recommend a conservative approach, except for very high risk patients.1,3 Although the procedure is controversial due to the risk of dissection propagation, 13 patients in our study underwent invasive reassessment, eight due to pain recurrence and five due to operator decision. Coronary CT angiography is an attractive non-invasive alternative for diagnosing SCAD, however its lack of resolution for small vessels limits visualization of the distal portion of vessels, which is frequently affected by SCAD.2 Of those undergoing repeat angiography during hospitalization, half presented disease progression, whereas all elective coronary angiographies performed during follow-up showed disease improvement.
Regarding medical therapy, expert consensus suggests that dual antiplatelet therapy may be considered during the acute phase and for up to one year for patients who receive medical therapy.2 Statins are not routinely recommended in SCAD, since it is not commonly mediated by atherosclerotic plaque rupture and data show conflicting results.3,12,16 Of note, a study by Saw et al. associated the use of beta-blockers with a decrease in the incidence of recurrent SCAD.16 In our center, almost all patients were discharged on aspirin, the majority concomitantly on clopidogrel, and a significant proportion of patients were medicated with beta-blockers and RASi. Patients who had concomitant left ventricular thrombus or atrial fibrillation were prescribed anticoagulation, and statins were prescribed for primary prevention.
The incidence of recurrent SCAD, defined as a new dissection event temporally separated from the index event,2 ranges from 10% to 30%, depending on studies and time to follow-up.7 In our population, we defined SCAD recurrence as a new ischemic event during follow-up; it was diagnosed in five patients (all NSTEMI), in four of whom a new SCAD was well documented.
No patients died during follow-up, which once more confirms the low mortality rate in SCAD.2 No association was found between the use of beta-blockers, RASi, statins or percutaneous interventions and SCAD recurrence during follow-up. Of note, our study was not powered to identify correlations between treatment and major outcomes, due to its relatively small sample size and the low incidence of major adverse outcomes during follow-up. Coronary artery tortuosity is highly prevalent in SCAD and was associated with recurrent events,17 however no particular type of dissection has been specifically associated with a higher risk of events. In our study, we found that patients with type 1 dissection presented a significantly higher rate of SCAD recurrence in a median follow-up of approximately three years.
Study limitationsThe principal limitation of this study was its relatively small sample size with a low incidence of major adverse outcomes during follow-up, which precludes major conclusions regarding associations and/or correlations. Also, due to misdiagnosis and underdiagnosis of SCAD, this cohort may not represent all patients with SCAD. Other limitations include the retrospective and observational nature of the analysis and the fact that some data were missing.
ConclusionIn agreement with other series of patients diagnosed with SCAD, our patients were mainly young or middle-aged women, frequently with conditions associated with coronary artery dissection. The LAD and mid-distal segments were the most affected vessels and type 2 dissection was the most prevalent type. No association was found between the use of beta-blockers, RASi, statins or percutaneous interventions and SCAD recurrence during follow-up. Importantly, for the first time our study reported a correlation between type 1 SCAD and a significantly higher rate of SCAD recurrence at long-term follow-up. Altogether, these results highlight the need for larger long-term follow-up studies and randomized controlled trials in order to optimize management and care in this population.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Marta Silva and Roberto Pinto for their work on reviewing coronary angiograms.