Obstructive sleep apnea (OSA) is one of the main risk factors for cardiovascular diseases and is associated with both morbidity and mortality. OSA has also been linked to arrhythmias and sudden death.
ObjectiveTo assess whether OSA increases the risk of sudden death in the non-cardiac population.
MethodsThis is a systematic review of the literature. The descriptors “sudden death” and “sleep apnea” and “tachyarrhythmias” and “sleep apnea” were searched in the PubMed/Medline and SciELO databases.
ResultsThirteen articles that addressed the relationship between OSA and the development of tachyarrhythmias and/or sudden death with prevalence data, electrocardiographic findings, and a relationship with other comorbidities were selected. The airway obstruction observed in OSA triggers several systemic repercussions, e.g., changes in intrathoracic pressure, intermittent hypoxia, activation of the sympathetic nervous system and chemoreceptors, and release of catecholamines. These mechanisms would be implicated in the appearance of arrhythmogenic factors, which could result in sudden death.
ConclusionThere was a cause–effect relationship between OSA and cardiac arrhythmias. In view of the pathophysiology of OSA and its arrhythmogenic role, studies have shown a higher risk of sudden death in individuals who previously had heart disease. On the other hand, there is little evidence about the occurrence of sudden death in individuals with OSA and no heart disease, and OSA is not a risk factor for sudden death in this population.
A apneia obstrutiva do sono (AOS) é um dos principais fatores de risco para doenças cardiovasculares e está associada a relevante morbimortalidade. A AOS também tem sido relacionada com o desenvolvimento de arritmias e morte súbita.
ObjetivoRelacionar a AOS com o aumento do risco de morte súbita na população não cardiopata.
MétodosTrata-se de revisão sistemática da literatura. Foi realizada a pesquisa dos descritores “sudden death” and “sleep apnea” e “tachyarrhythmias” and “sleep apnea” nas plataformas Pubmed/Medline e SciELO.
ResultadosForam selecionados 13 estudos que abordavam a relação entre AOS e o desenvolvimento de taquiarritmias e/ou morte súbita com dados de prevalência, achados eletrocardiográficos e relação com outras comorbidades. A obstrução das vias aéreas observada na AOS desencadeia diversas repercussões sistémicas, como, por exemplo, alterações da pressão intratorácica, hipóxia intermitente, ativação do sistema nervoso simpático, ativação de quimiorreceptores e liberação de catecolaminas. Esses mecanismos estariam implicados no surgimento de fatores arritmogênicos, que poderiam implicar a ocorrência de morte súbita.
ConclusõesVerificou-se existência de relação causa-efeito entre AOS e arritmias cardíacas. Diante da fisiopatologia da AOS, no que tange ao seu papel arritmogênico, estudos demonstram maior risco de morte súbita em indivíduos previamente cardiopatas. Em contrapartida, existem poucas evidências sobre a ocorrência de morte súbita em indivíduos com AOS não cardiopatas, não se configurando a AOS por si um fator de risco para morte súbita nesta população.
Sleep breathing disorders are mainly characterized by nocturnal hypoxia and are significant among the diseases affecting the adult population.1 Among these disorders, obstructive sleep apnea (OSA) is characterized by the repetitive obstruction of the upper airway during sleep, causing snoring, breathing cessation, daytime sleepiness, and fatigue.2–5
Studies show that 3.7–49.7% of the world population has more than five apnea and hypopnea events per hour of sleep, with this wide variation being partly due to the absence of homogeneity in epidemiological studies.6,7 In the HypnoLaus cohort study with 2121 participants, the prevalence of moderate-to-severe sleep-disordered breathing (≥15 events per hour) was 23.4% in women and 49.7% in men. Hypopnea represented the most common respiratory event (75%), followed by OSA (19%), central apnea (4%), and mixed apnea (2%).7 In another epidemiological study conducted in the city of São Paulo, the results revealed that a third of the inhabitants (32.8%) met the criteria for OSA diagnosis.6 This disorder is prevalent in both sexes and in any age or weight group. However, the main profile of individuals diagnosed with sleep apnea consists of male, obese, and elderly patients.8
OSA is considered one of the main risk factors for cardiovascular diseases. Among patients who receive medical care due to cardiovascular events, more than 65% are diagnosed with OSA.9,10 Moreover, OSA is also associated with excessive cardiovascular morbimortality, including sudden cardiac death during sleep.11
Sudden death is defined as a nontraumatic, unexpected, fatal event that occurs within one hour of the onset of symptoms in an apparently healthy individual.12,13 In the USA, sudden cardiac death (SCD) is estimated to cause 180000–400000 deaths per year, most of which are related to the incidence of coronary artery disease. In Brazil, SCD mortality affects approximately 48/100000 inhabitants. Men between the sixth and seventh decade of life are the individuals most affected by SCD.14
Recent scientific literature contains significant evidence on the relationship between OSA and sudden death in subjects with previous cardiovascular diseases, e.g., heart failure and hypertension. However, few available studies address the relevance of this sleep disorder as a risk factor for sudden death in the general population.15 From this perspective, this study aimed to assess whether OSA was associated with an increased risk of sudden death in the non-cardiac population.
MethodsThis study consisted of a systematic review of the literature following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement16 aiming to answer the following question: “does OSA increase the risk of sudden death in the non-cardiac population?”
The research question was constructed using the PICOS strategy (Table 1).
PICOS strategy for searching the literature.
| Population | Patients diagnosed with OSA*. |
| Intervention | Risk of sudden death. |
| Comparison | Patients with OSA and previous cardiovascular diseases under the risk of sudden death. |
| Outcomes | Development of sudden death; development of tachyarrhythmias. |
| Study design | Original articles and meta-analyses. |
The review included original articles and meta-analyses from 2015 to 2021 that addressed the relationship between OSA and sudden death and/or tachyarrhythmias, published in English, Spanish, or Portuguese.
Studies whose central subject was not the relationship between sleep apnea and the development of sudden death and/or tachyarrhythmias were excluded from the process. Studies involving animals and articles whose full version was not available were also excluded.
Search strategy and study selectionThe MEDLINE® via PubMed® and SciELO databases were searched for articles published until March 4, 2021 using the descriptors “sudden death” and “sleep apnea” and “tachyarrhythmias” and “sleep apnea”. Original articles and meta-analyses published from 2015 to 2021 in the national and international literature were selected. The descriptors used are listed in the Medical Subject Headings terms (MeSH) of the US National Library of Medicine (NLM).
The search strategy used for the MEDLINE® via PubMed® database was as follows:
- 1.
(“tachyarrhythmias”[All Fields] AND “sleep apnea”[All Fields])
- 2.
(“tachyarrhythmias”[All Fields] AND “sleep apnea”[All Fields]) AND (2015:2021[pdat])
- 3.
(“sudden death”[All Fields] AND “sleep apnea”[All Fields])
- 4.
(“sudden death”[All Fields] AND “sleep apnea”[All Fields]) AND (2015:2021[pdat])
The methodological selection was descriptive, independent, and performed by two reviewers. Initially, the titles and abstracts of the articles were evaluated according to the eligibility and exclusion criteria. Then, the selected articles were read in full for a new evaluation according to the inclusion and exclusion criteria.
Data extraction and analysisThe selected articles were analyzed descriptively, and two independent reviewers performed data extraction by listing the main results and conclusions that addressed the relationship between OSA and the development of sudden death and/or tachyarrhythmias.
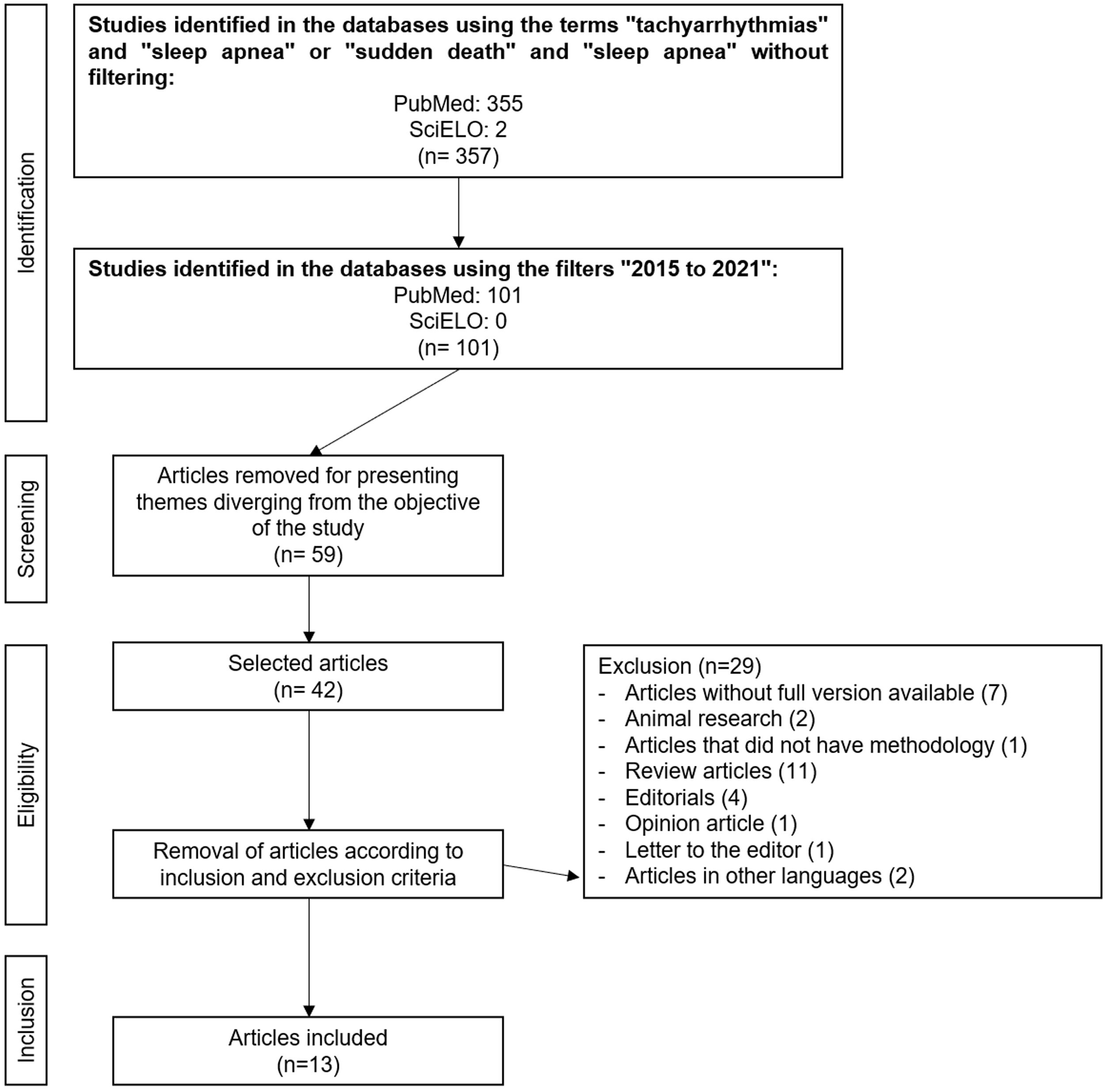
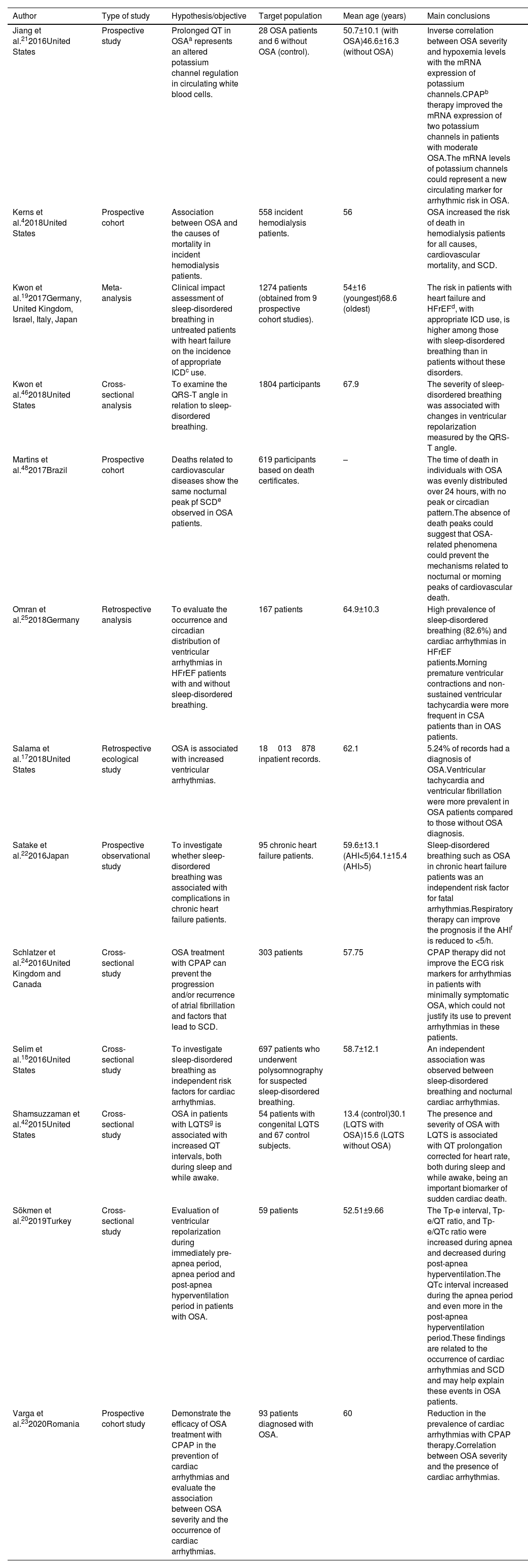
ResultsAfter the initial search using English descriptors and no filters, 355 articles were retrieved from PubMed® and only two from SciELO, resulting in 357 studies. When applying the time filter to search for articles published from 2015 to 2021, the number of studies was reduced to 101, all from PubMed®. After reading the titles, abstracts, and full texts, 59 articles were excluded for not addressing a theme related to the objective of this study. Of the 42 remaining articles, 29 were excluded according to the pre-established inclusion and exclusion criteria, totaling a final sample of 13 articles. Figure 1 describes this process.
The location with the highest number of studies (n=6) was the USA, while the others were conducted in Turkey, Brazil, Germany, the United Kingdom, Canada, Japan, Italy, Israel, and Romania. The mean age of the subjects ranged from 13.4 to 68.6 years. Most studies (n=8) showed more male participants than females. Except for one study based on death certificates, all others were performed with live patients or with medical record data (Table 2).
Data of the study included in the review.
| Author | Type of study | Hypothesis/objective | Target population | Mean age (years) | Main conclusions |
|---|---|---|---|---|---|
| Jiang et al.212016United States | Prospective study | Prolonged QT in OSAa represents an altered potassium channel regulation in circulating white blood cells. | 28 OSA patients and 6 without OSA (control). | 50.7±10.1 (with OSA)46.6±16.3 (without OSA) | Inverse correlation between OSA severity and hypoxemia levels with the mRNA expression of potassium channels.CPAPb therapy improved the mRNA expression of two potassium channels in patients with moderate OSA.The mRNA levels of potassium channels could represent a new circulating marker for arrhythmic risk in OSA. |
| Kerns et al.42018United States | Prospective cohort | Association between OSA and the causes of mortality in incident hemodialysis patients. | 558 incident hemodialysis patients. | 56 | OSA increased the risk of death in hemodialysis patients for all causes, cardiovascular mortality, and SCD. |
| Kwon et al.192017Germany, United Kingdom, Israel, Italy, Japan | Meta-analysis | Clinical impact assessment of sleep-disordered breathing in untreated patients with heart failure on the incidence of appropriate ICDc use. | 1274 patients (obtained from 9 prospective cohort studies). | 54±16 (youngest)68.6 (oldest) | The risk in patients with heart failure and HFrEFd, with appropriate ICD use, is higher among those with sleep-disordered breathing than in patients without these disorders. |
| Kwon et al.462018United States | Cross-sectional analysis | To examine the QRS-T angle in relation to sleep-disordered breathing. | 1804 participants | 67.9 | The severity of sleep-disordered breathing was associated with changes in ventricular repolarization measured by the QRS-T angle. |
| Martins et al.482017Brazil | Prospective cohort | Deaths related to cardiovascular diseases show the same nocturnal peak pf SCDe observed in OSA patients. | 619 participants based on death certificates. | – | The time of death in individuals with OSA was evenly distributed over 24 hours, with no peak or circadian pattern.The absence of death peaks could suggest that OSA-related phenomena could prevent the mechanisms related to nocturnal or morning peaks of cardiovascular death. |
| Omran et al.252018Germany | Retrospective analysis | To evaluate the occurrence and circadian distribution of ventricular arrhythmias in HFrEF patients with and without sleep-disordered breathing. | 167 patients | 64.9±10.3 | High prevalence of sleep-disordered breathing (82.6%) and cardiac arrhythmias in HFrEF patients.Morning premature ventricular contractions and non-sustained ventricular tachycardia were more frequent in CSA patients than in OAS patients. |
| Salama et al.172018United States | Retrospective ecological study | OSA is associated with increased ventricular arrhythmias. | 18013878 inpatient records. | 62.1 | 5.24% of records had a diagnosis of OSA.Ventricular tachycardia and ventricular fibrillation were more prevalent in OSA patients compared to those without OSA diagnosis. |
| Satake et al.222016Japan | Prospective observational study | To investigate whether sleep-disordered breathing was associated with complications in chronic heart failure patients. | 95 chronic heart failure patients. | 59.6±13.1 (AHI<5)64.1±15.4 (AHI>5) | Sleep-disordered breathing such as OSA in chronic heart failure patients was an independent risk factor for fatal arrhythmias.Respiratory therapy can improve the prognosis if the AHIf is reduced to <5/h. |
| Schlatzer et al.242016United Kingdom and Canada | Cross-sectional study | OSA treatment with CPAP can prevent the progression and/or recurrence of atrial fibrillation and factors that lead to SCD. | 303 patients | 57.75 | CPAP therapy did not improve the ECG risk markers for arrhythmias in patients with minimally symptomatic OSA, which could not justify its use to prevent arrhythmias in these patients. |
| Selim et al.182016United States | Cross-sectional study | To investigate sleep-disordered breathing as independent risk factors for cardiac arrhythmias. | 697 patients who underwent polysomnography for suspected sleep-disordered breathing. | 58.7±12.1 | An independent association was observed between sleep-disordered breathing and nocturnal cardiac arrhythmias. |
| Shamsuzzaman et al.422015United States | Cross-sectional study | OSA in patients with LQTSg is associated with increased QT intervals, both during sleep and while awake. | 54 patients with congenital LQTS and 67 control subjects. | 13.4 (control)30.1 (LQTS with OSA)15.6 (LQTS without OSA) | The presence and severity of OSA with LQTS is associated with QT prolongation corrected for heart rate, both during sleep and while awake, being an important biomarker of sudden cardiac death. |
| Sökmen et al.202019Turkey | Cross-sectional study | Evaluation of ventricular repolarization during immediately pre-apnea period, apnea period and post-apnea hyperventilation period in patients with OSA. | 59 patients | 52.51±9.66 | The Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio were increased during apnea and decreased during post-apnea hyperventilation.The QTc interval increased during the apnea period and even more in the post-apnea hyperventilation period.These findings are related to the occurrence of cardiac arrhythmias and SCD and may help explain these events in OSA patients. |
| Varga et al.232020Romania | Prospective cohort study | Demonstrate the efficacy of OSA treatment with CPAP in the prevention of cardiac arrhythmias and evaluate the association between OSA severity and the occurrence of cardiac arrhythmias. | 93 patients diagnosed with OSA. | 60 | Reduction in the prevalence of cardiac arrhythmias with CPAP therapy.Correlation between OSA severity and the presence of cardiac arrhythmias. |
After analyzing the national database of inpatients in the United States, Salama and others identified that 2.24% of OSA patients had one episode of ventricular tachycardia compared to 1.16% of the control group. Furthermore, ventricular fibrillation was more prevalent in the OSA group (0.3%) than in the group without OSA (0.2%). It is worth noting that this study was conducted with a sample of 18013878 inpatients from 2012 to 2014.17 In a cross-sectional analysis with 697 subjects, patients with moderate–severe sleep-disordered breathing (SDB) had almost threefold unadjusted odds of any cardiac arrhythmia and twofold odds of tachyarrhythmias than patients without SDB.18 Moreover, the results of a meta-analysis highlight the role of arrhythmogenicity as an important mediating mechanism in the relationship between sleep-disordered breathing and the prognosis of patients with heart failure.19
Sökmen et al. investigated the electrocardiographic aspects of a population with obstructive sleep apnea. They noted that the Tp-e and QT intervals and the Tp-e/QT and Tp-e/QTc ratios increased significantly during the apnea period compared to the period immediately before its beginning. Prolonged QT, QTc, and Tp-e intervals are indicators of lethal ventricular arrhythmias.20 In another study with 28 OSA patients and six patients without OSA, the authors stated that the levels of the potassium channel gene could represent a new circulating marker for arrhythmic risk in OSA. The mRNA expression of most potassium channels was inversely correlated with OSA severity, suggesting that QT prolongation can be caused by loss of function in the potassium channels. Therefore, the greater the severity of OSA, the lower the expression of potassium channels and the higher the odds of QT prolongation, leading to severe arrhythmias.21
In a study conducted with 95 chronic heart failure patients, the prevalence of fatal arrhythmic events during sleep was 0% in patients with an apnea–hypopnea index (AHI) <5/h and 44% in patients with AHI ≥5/h. Eight ventricular tachyarrhythmias occurred during the follow-up period. Due to this, the study concluded that an AHI ≥5 is an important risk factor for fatal arrhythmic events.22 In a study conducted with incident hemodialysis patients, 12% of which had an OSA diagnosis, this group showed a threefold higher risk of sudden death, characterizing OSA as a significant risk factor for mortality among dialysis patients.4
Varga et al. studied the impact of a continuous positive airway pressure device (CPAP) on the treatment of cardiac arrhythmias in 93 OSA patients. The authors observed that this disorder was more commonly associated with atrial fibrillation, ventricular extrasystoles, non-sustained ventricular tachycardia, and sinus pauses. CPAP therapy was effective in treating both sleep disorders and cardiac arrhythmia.23 On the other hand, Schlatzer et al. found no evidence of the impact of CPAP use on any electrocardiographic risk marker for arrhythmias.24
Finally, in a retrospective analysis with 167 patients, Omran et al. observed that central sleep apnea (CSA) was associated with ventricular tachycardia episodes at any time of the day or night in patients with heart failure. In contrast, OSA patients tend to experience arrhythmic episodes during sleep.25
DiscussionPathophysiology of sleep apneaSleep apnea is characterized by repetitive ventilation interruptions longer than or equal to 10 seconds during sleep and is associated with an ongoing ventilatory effort by the affected subject.26 In turn, hypopnea is characterized by an airflow reduction for ten or more seconds associated with a drop in oxyhemoglobin saturation or electroencephalographic arousal.27
Apneas can be classified as central (CSA) and obstructive (OSA). OSA occurs due to upper airway occlusion. In contrast, CSA is related to changes in the nervous system responsible for generating the breathing rhythm.27 Sleep apnea can also be classified according to the severity of the disorder using the AHI, representing the number of apnea and hypopnea episodes per hour of sleep.28 OSA is considered mild with an AHI from 5 to 15/h, moderate with an AHI from 15 to 30, and severe with an AHI >30.29
The activity of the genioglossus muscle decreases during sleep, causing the tongue to fall backward and obstruct the airways of susceptible individuals. Obesity is the main predisposing factor for sleep apnea due to direct mechanical effects that result in reduced caudal traction of the upper airway, compromising its patency due to the deposition of fat within the upper airway and lung volume reduction.27
The basic mechanism of OSA pathophysiology is related to several factors, e.g., age and sex, and it has shown to be more prevalent among older men. Posture and gravity also contribute to the development of this disorder, especially due to the decubitus position during sleep. Finally, genetic, functional, and anatomical factors are also important causes of OSA due to morphological abnormalities in the oral cavity and the muscles of this region.30
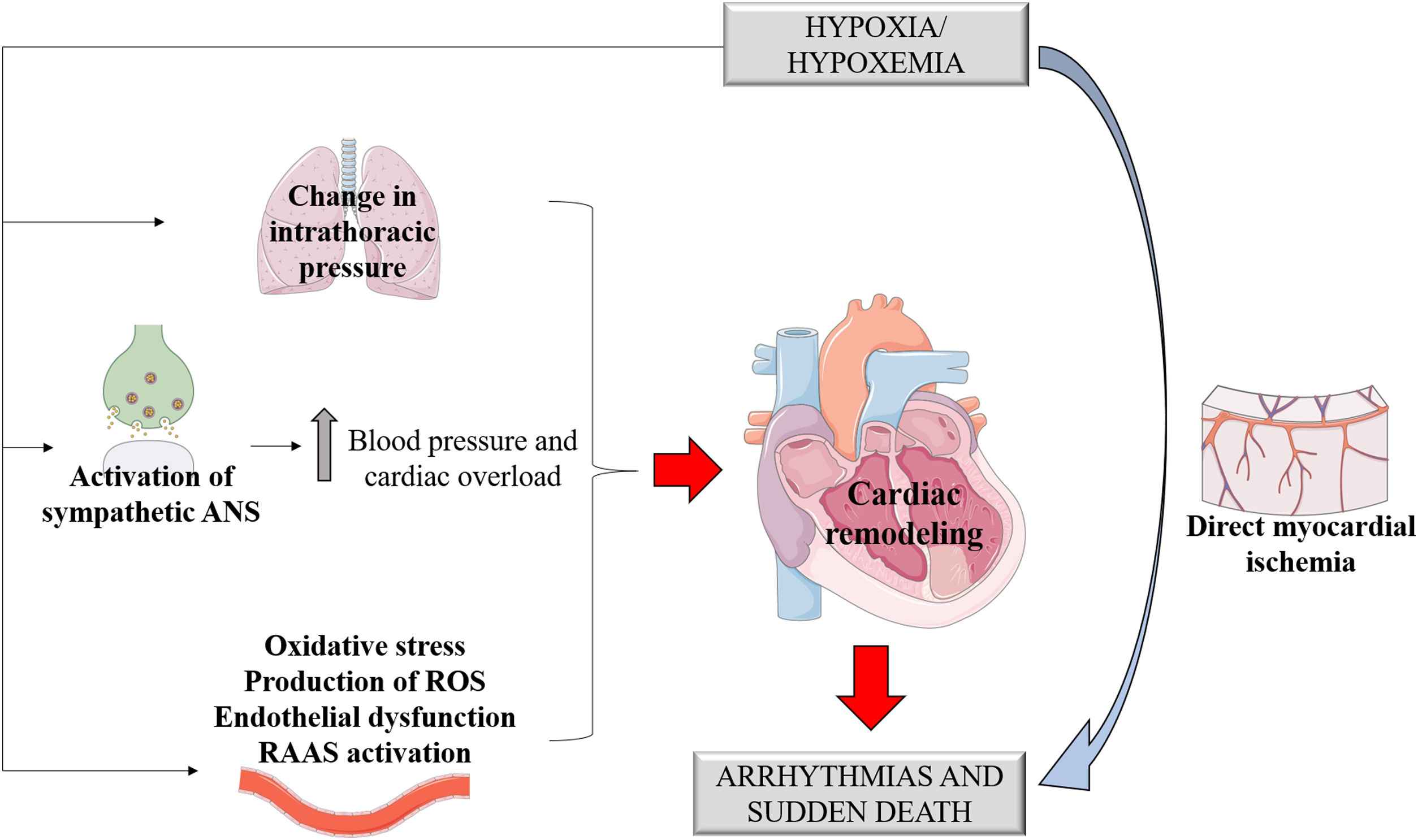
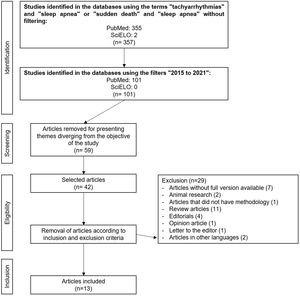
Sleep-disordered breathing is related to several pathological mechanisms such as changes in intrathoracic pressure, intermittent hypoxia and injury, sympathetic nervous system activation, sleep reduction and fragmentation, cardiac remodeling, metabolic dysregulation, and endothelial dysfunction (Figure 2).12,31,32
In addition to endothelial dysfunction, the inflammatory mechanisms unleashed by OSA can also increase atherosclerosis, contributing to the progression of cardiovascular diseases. It has also been described that OSA patients without other risk factors for arteriosclerosis showed increased intima-media thickness compared to patients without OSA, which is related to nocturnal hypoxia.33
Sleep-stage dependent changes in respiration, heart rate, and blood pressure normally occur during sleep. However, in OSA and CSA cases, these variations are more correlated with the severity of the disorder and the duration of airflow restriction.34 Physiologically, non-REM sleep (rapid eye movement) occupies most of the sleep architecture and is characterized by the stability of the autonomic nervous system, with heightened parasympathetic tone and lower activity of the sympathetic tone,32 keeping the heart at a low rate and maintaining low blood pressure levels.27 However, in individuals with OSA, chemoreceptors and baroreceptors are activated in response to the collapse of the upper airways and reduced partial pressure of oxygen. These receptors stimulate catecholamine release by the sympathetic autonomic nervous system, resulting in increased blood pressure and heart overload,12 implying OSA as a possible factor in the genesis of arterial hypertension. Therefore, this apnea-induced sympathetic response to hypoxia and hypercapnia could explain the increased risk of cardiovascular diseases and death among OSA patients.34
Sleep apnea and arrhythmiasPatients with OSA have an increased risk of developing cardiac arrhythmias, the most prevalent of which is atrial fibrillation (AF) arrhythmia.12,35 The European Society of Cardiology highlights OSA as a risk factor for AF.36,37 Several pathophysiological mechanisms unleashed by OSA have been related to arrhythmogenesis, e.g., catecholamine release in the ischemic myocardium.11 In the long run, the hypertension generated, as well as endothelial dysfunction and ventricular remodeling caused by exposure to the sympathetic tone, would also be implied.32
Changes in intrathoracic pressure are also important pre-arrhythmogenic effects. Increased intrathoracic pressure and respiratory effort stimulate cardiac mechanoreceptors that promote changes in cardiac geometry. By themselves, these changes can generate arrhythmias by electrical feedback mechanisms.12 It is worth noting that cardiac remodeling represents the formation of fibrous tracts that act as obstacles to electrical conduction. Therefore, this constant electrical and anatomical remodeling is a perpetuating factor of arrhythmias.38
Heart rate variability could represent the sympathetic activity resulting from apnea or hypopnea events. However, an observational study conducted by Xie et al. under the hypothesis that the prevalence of cardiac arrhythmias is associated with OSA and that heart rate variability parameters could be effective markers for OSA screening, found no difference in the parameters between OSA patients and the control group, suggesting that the ECG alone is not an effective screening method, even though severe obstructive sleep apnea is an important risk factor for arrhythmias.39
With regard to the differences between the types of sleep apnea in the assessment of the circadian distribution of arrhythmias in patients with sleep-disordered breathing, CSA predominated in the studied population compared to OSA, and the association of heart failure with reduced ejection fraction (HFrEF) and ventricular arrhythmias was more frequent among CSA patients. With regard to the differences in the underlying mechanisms that drive and promote arrhythmias caused by OSA in relation to CSA, arrhythmias in OSA patients occur during apnea moments due to oxygen desaturation, increased intrathoracic pressure, and myocardial wall stress. Conversely, in CSA, hypopneas cause intrathoracic pressure swings, pH displacements, changes in oxygen and carbon dioxide partial pressures, and increased circulatory delay. Moreover, differences in the time profiles of these arrhythmias may occur because OSA patients experience such events during sleep, whereas CSA patients might experience them while awake.25
Atrial fibrillation, a type of supraventricular arrhythmia, is more common among CSA patients, corroborating the hypothesis that different arrhythmias may be linked to different types of sleep apnea-related stresses (OSA or CSA).34 In experiments with pig models to assess the differences between OSA and CSA on the origin of ventricular repolarization changes, it was observed that intrathoracic pressure swings during obstructive apneas are associated with prolonged RT and Tp-e intervals, which was not significantly observed in central apneas.40
Although more common in individuals with CSA, the relationship between OSA and atrial fibrillation is well known in the scientific community, with OSA being an independent risk factor for atrial fibrillation. Furthermore, the increased risk was directly correlated with the severity of apnea and the recurrence and worsening of the arrhythmia prognosis.41
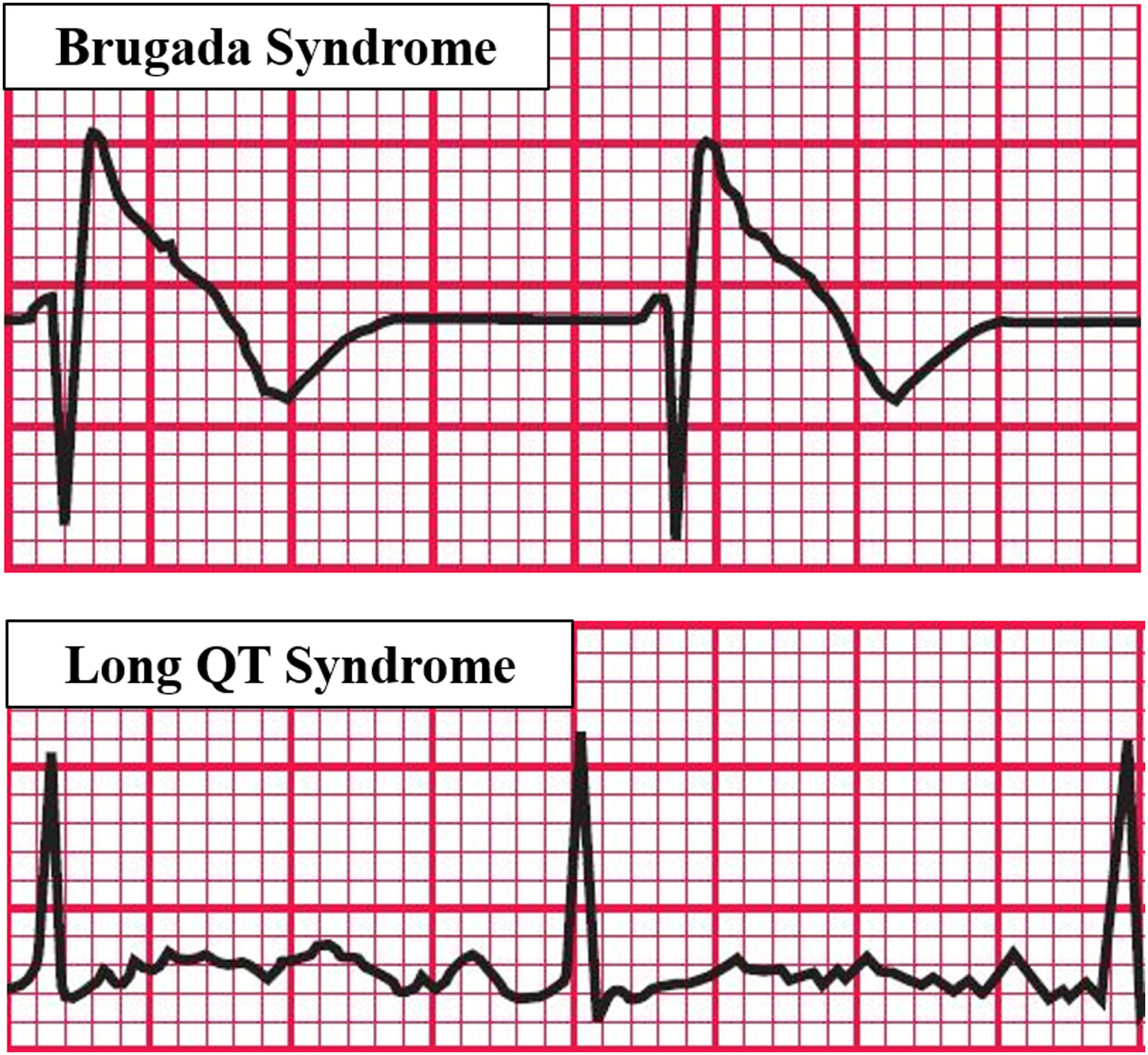
The relationship between OSA and complex ventricular ectopy has also been described, tending to increase with OSA severity. High OSA prevalence was found among patients with Brugada syndrome, a condition known to be potentially fatal, raising the possibility that the association of the two disorders could lead to increased mortality in this group of patients.34
Prolonged QT, QTc, and Tp-e intervals could imply changes in ventricular repolarization, causing ventricular arrhythmias. A significant prolongation of the QTc interval was reported by Sökmen et al. during apnea, which was maintained in the post-apnea hyperventilation period. The Tp-e interval, whose increase was observed in the apnea periods of this study, is a recent parameter of cardiac repolarization whose prolongation could be associated with ventricular arrhythmias and SCD. On the other hand, Tp-e/QT is a ventricular repolarization index that could be a more stable and, therefore, more accurate predictor of ventricular arrhythmias.20
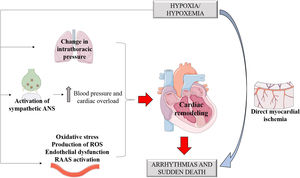
Experiments that studied the association between OSA and Long QT syndrome (LQTS) (Figure 3) evidenced that the association of the two diseases increased respiratory disturbances, AHI, and the arousal index. Furthermore, QTc increased both during sleep and while awake among these patients, with an association between apnea severity and QT prolongation corrected for heart rate, constituting an important biomarker of sudden death. The study suggests that OSA treatment in LQTS patients could reduce the QTc and thus reduce the risk of sudden death.42
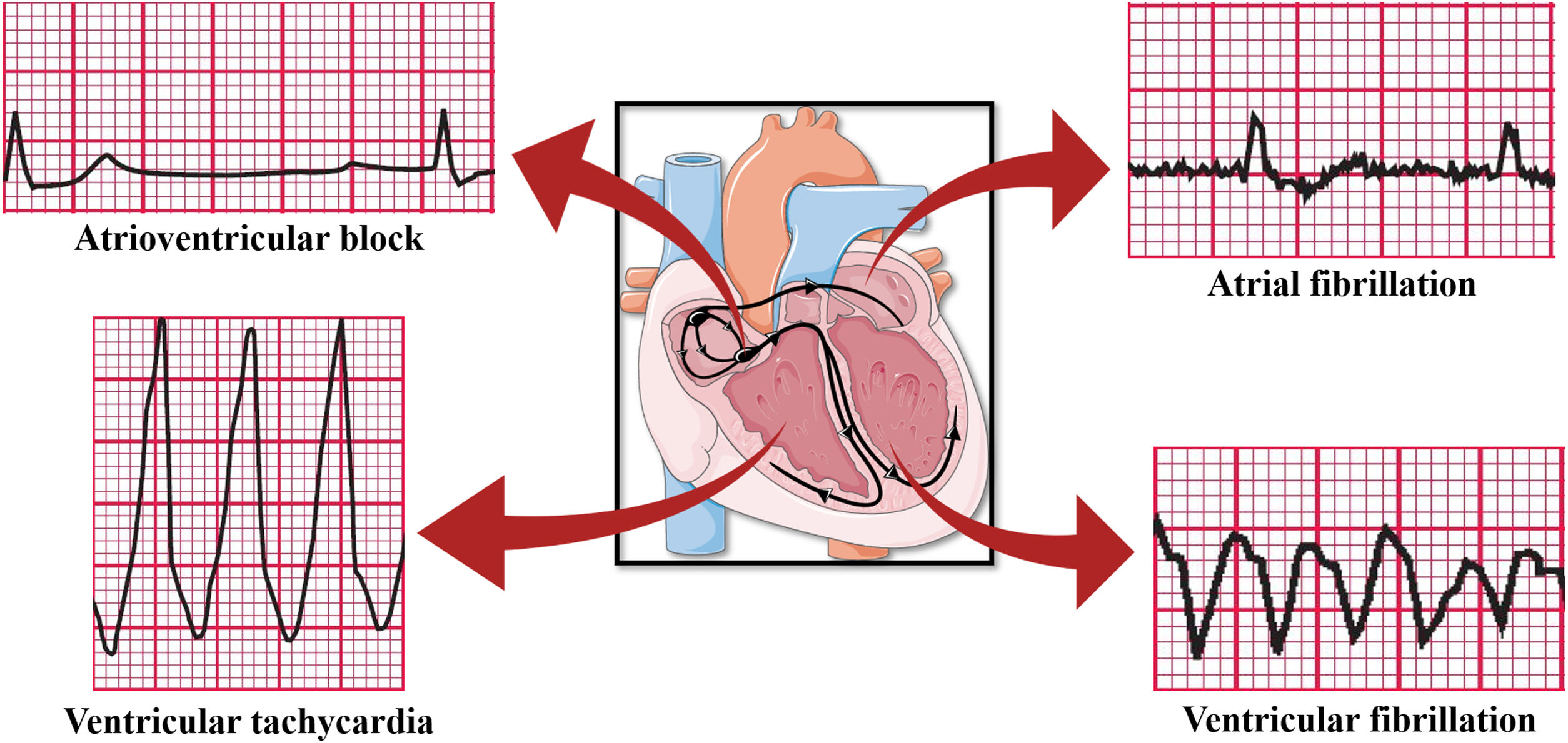
Predisposing factors for arrhythmias associated with OSA. Based on electrocardiographic tracings of Hampton, 2013.43
Regarding ventricular arrhythmias, Salama et al. studied the relationship between OSA and increased arrhythmias and concluded that ventricular tachycardia and ventricular fibrillation are more prevalent among OSA patients than among patients without an OSA diagnosis.17
Considering the relationship between OSA and the increased risk of ventricular arrhythmias and conduction disturbances, it has been reported that OSA patients showed a combination of changes in the QRS complex, e.g., a leftward shift of the electrical axis and low QRS voltage, suggesting a deterioration of the depolarization sequence possibly related to electrical remodeling. Furthermore, these changes become more important with the severity of obstructive apnea.5
In experiments with rats to investigate the effects of intermittent hypoxia on the development and severity of myocardial ischemia-related ventricular arrhythmias, it was possible to observe an increase in the incidence of ischemic arrhythmias, particularly ventricular fibrillation, in addition to QTc prolongation. These findings highlight the role of intermittent hypoxia as an important risk factor for SCD through mechanisms such as myocardial ischemia, sympathetic activation, and alterations in ventricular repolarization.11
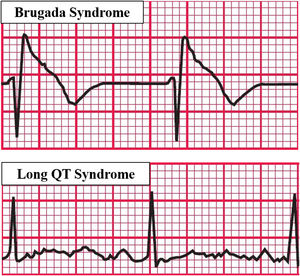
In addition to the already reported ventricular arrhythmias and atrial fibrillation, apnea and hypoxia induced by OSA could lead to vagal activation and cause bradyarrhythmia (Figure 4)43 due to the cardiac vagal activation resulting from apneic and hypoxic episodes even if the increase in the sympathetic tone, also resulting from hypoxia, is implied in the origin of tachyarrhythmias. There would be a large distribution of the R-R interval with the presence of contrary stimuli resulting from vagal and sympathetic activation. Yajima et al. reported the case of a patient with sinus arrest and atrioventricular block accompanied by OSA. Since the patient could not be treated with pacemaker therapy, conventional OSA treatment by continuous positive airway pressure (CPAP) was shown to be effective, decreasing the plasma brain natriuretic peptide levels and highlighting the reduction in cardiac overload caused by bradyarrhythmia and the change in the distribution of the R-R interval, which was close to normal, demonstrating the balance of the autonomic nervous system and suggesting that CPAP therapy could effectively control cardiac arrhythmias in OSA patients.44
Arrhythmias caused by OSA. Based on electrocardiographic tracings of Hampton, 2013.43
Sudden death is a nontraumatic, unexpected, fatal event that occurs within one hour of the onset of the first symptoms in previously healthy individuals. The conditions established by the European Society of Cardiology for sudden death characterization are the presence of a congenital or acquired potentially fatal cardiac condition, autopsy identifying a cardiac or vascular anomaly as the probable cause of the event, and no obvious extracardiac causes, suggesting an arrhythmic event as the likely cause of death. These parameters highlight the lethal potential of cardiac arrhythmias and the consequent implication of OSA.12
Several OSA-stimulated biological pathways lead to cardiac electrical abnormalities and increase the susceptibility to arrhythmias, including ventricular arrhythmias. Ventricular fibrillation is among ventricular arrhythmias and is the main arrhythmia associated with sudden death. It is also known that sudden death occurs predominantly during sleeping hours in individuals with OSA.45 Increased sympathetic tone, intermittent hypoxia, and intrathoracic pressure swings are described as the main changes that explain the association between OSA and increased arrhythmogenesis propensity, also explaining the occurrence of nocturnal arrhythmias in patients with OSA.45
In addition to the apnea/hypopnea index, nocturnal hypoxia constitutes an independent risk factor for SCD.46 Furthermore, there is a relationship between the oxygen saturation threshold <93% and a 2.9 times higher risk of SCD.12 Chronic exposure to hypoxia alters the left ventricular transmural action potential gradient. This alteration is responsible for QT prolongation in the electrocardiographic tracing and constitutes a risk factor for arrhythmias and sudden death.11
Organic changes due to hypoxemia, e.g., oxidative stress, production of reactive oxygen species, endothelial dysfunction, and activation of the renin-angiotensin-aldosterone system, lead to ventricular remodeling due to arrhythmogenesis.3 However, myocardial ischemia resulting from hypoxemia can also lead to sudden death and is one of its primary causes.11 Studies also suggest that, in addition to myocardial ischemia, fibrosis and wall stress due to hypertension after sympathetic stimulation favor modifications in the depolarization waves and reentry mechanisms, increasing the risk of ventricular arrhythmias and sudden death.47
Although including the QRS complex (a component of ventricular depolarization), the prolongation of the QT interval, which mainly represents ventricular repolarization, is described as a risk factor often associated with sudden death.24 It has also been reported that, in OSA patients, the QT interval increases with the severity of the disorder. Furthermore, QT prolongation reflects the increased heterogeneity of ventricular repolarization and the presence of ventricular tachycardia.38
In the analysis of 619 death certificates of adults who underwent polysomnography, of which 160 resulted in cardiovascular-related deaths, patients without OSA and the general population showed lower mortality rates during the night, while OSA patients showed a uniform distribution of cardiovascular death during daytime hours. Furthermore, in the general population and patients without OSA, there was a peak of sudden death in the early morning hours and a nocturnal decrease, which was not seen among patients with obstructive apnea. In this study, 23% of deaths among OSA patients occurred in the morning and 23% at night.48 In turn, a previous study observed 46% of sudden deaths occurring at night and 20% in the morning among patients with obstructive apnea. However, this study observed that the AHI of people with SCD during sleeping hours was higher than among those who died at other times of the day, demonstrating the direct relationship between AHI and the risk of SCD during sleep, differing from the general population, with a nocturnal nadir and a morning peak.49
A meta-analysis of 13 studies highlighted the association between sleep apnea and fatal events, cardiovascular outcomes, and hospitalization time, suggesting that the treatment of sleep apnea could mitigate these indicators.50 However, a study that compared patients with severe obstructive sleep apnea (AHI >30) and a control group of patients with AHI <5 showed that the QTc interval of patients with severe apnea was more dispersed than in the control group, highlighting a significant improvement among these patients after CPAP therapy, which could reduce the risk of arrhythmias and cardiovascular events.51
Finally, a study conducted for six months with 303 patients with minimally symptomatic obstructive sleep apnea using ECG risk markers analyzed the role of CPAP therapy on the behavior of atrial fibrillation and the remaining factors that could lead to sudden death, observing no effect of CPAP therapy on the risk markers for arrhythmias and sudden death in this group.24
ConclusionsThere was a cause–effect relationship between OSA and cardiac arrhythmias. There is a significant occurrence of sudden death among OSA individuals with previous heart diseases due to pathophysiological factors related to cardiac structural changes associated or not with arterial hypertension.3,4,12,28,45
In view of the pathophysiology of OSA and its arrhythmogenic role, studies have shown a higher risk of sudden death in individuals who previously had heart disease. There is, however, little evidence on the occurrence of sudden death in individuals with OSA and no heart disease, and OSA is not a risk factor for sudden death in this population.
Therefore, more studies and articles on this subject are required to validate this hypothesis since confirming that the OSA population without previous heart disease has an increased risk of sudden death would be fundamental to stimulate the development of health education and prevention strategies.
Conflicts of interestThe authors have no conflicts of interest to declare.