In obstructive hypertrophic cardiomyopathy (HCM), alcohol septal ablation (ASA) can lead to gradient reduction and symptom improvement. We aimed to assess the efficacy and safety of ASA in a long-term outcome study.
MethodsWe analyzed patients who underwent ASA over a seven-year period in a tertiary center. The primary echocardiographic endpoint was >50% reduction in left ventricular outflow tract (LVOT) gradient within a year of the procedure. The primary clinical endpoints were improvement in functional capacity and a combined endpoint of cardiac death and rehospitalization for cardiac cause. The follow-up period was 4.17±2.13 years.
ResultsA total of 80 patients, mean age 63.9±12.3 years, 30.0% male, were analyzed. Baseline LVOT gradient was 96.3±34.6 mmHg and interventricular septal thickness was 21.6±3.1 mm. Minor complications were observed in 6.3% and major complications in 2.5%, and 8.8% received a permanent pacemaker.
The primary echocardiographic endpoint was achieved by 85.7%. At three-month follow-up, LVOT gradient was 25.8±26.0 mmHg in the successful procedure group, compared to 69.2±35.6 mmHg in the other patients (p=0.001). At six months, LVOT gradient was 27.1±27.4 vs. 58.2±16.6 mmHg (p=0.024). Among 74 patients in NYHA class III/IV before the procedure, 57 (77%) improved to NHYA class I/II. The combined primary clinical endpoint (cardiac death and rehospitalization for cardiac cause) was observed in 27.5% (n=22). In the unsuccessful group, the combined endpoint was observed in 54.5%, compared to only 22.7% in the successful group. Only two patients died of cardiac causes.
ConclusionASA is a safe procedure with a high success rate. Patients who achieved significant reductions in LVOT gradient suffered less cardiac death and rehospitalization for cardiac cause.
Na miocardiopatia hipertrófica obstrutiva (MCHO), ablação septal alcoólica (ASA) pode levar a redução do gradiente e melhoria sintomática. O objetivo foi avaliar a eficácia e segurança da ASA no desfecho a longo prazo.
MétodosAnálise de doentes submetidos a ASA, durante sete anos, num centro terciário. Endpoint primário ecocardiográfico: redução >50% do gradiente na câmara de saída do ventrículo esquerdo (CSVE) durante o primeiro ano após o procedimento. Endpoints primários clínicos: a) melhoria da capacidade funcional; b) endpoint combinado: morte de causa cardíaca+hospitalização de causa cardíaca. Tempo de seguimento 4,17±2,13 anos.
ResultadosOitenta doentes, idade média de 63,9±12,3 anos, 30,0% homens. De base, o gradiente na CSVE era 96,3±34,6 mmHg, espessura do septo interventricular 21,6±3,1 mm. Complicações minor foram verificadas em 6,3%, complicações major em 2,5% e 8,8% receberam um pacemaker definitivo. O endpoint primário ecocardiográfico foi atingido em 85,7%. Aos três meses, o gradiente na CSVE foi de 25,8±26,0 mmHg no grupo com sucesso no procedimento contrastando com 69,2±35,6 mmHg nos restantes doentes (p=0,001). Aos seis meses, os gradientes na CSVE foram 27,1±27,4 versus 58,2±16,6 mmHg (p=0,024).
De entre os 74 doentes em classe NYHA III/IV antes do procedimento, 57 (77%) melhoraram para classe NYHA I/II. O endpoint primário combinado (morte de causa cardíaca+hospitalização de causa cardíaca) verificou-se em 27,5% (n=22). No grupo sem sucesso, o endpoint primário composto verificou-se em 54,5%, contrastando com apenas 22,7% no grupo com sucesso. Apenas dois doentes apresentaram morte de causa cardíaca.
ConclusãoA ASA é um procedimento seguro com elevada taxa de sucesso. Doentes que atingiram redução significativa do gradiente na CSVE apresentam menor morte de causa cardíaca e hospitalização de causa cardíaca.
Hypertrophic cardiomyopathy (HCM) is defined by the presence of unexplained asymmetric left ventricular (LV) hypertrophy associated with non-dilated ventricular chambers that cannot be explained by cardiac or systemic disease, including abnormal loading conditions.1,2 Its prevalence in the general population is estimated at approximately 1:500.3 However, recent information on its genetic basis in pathogenic mutations has led to the prevalence of HCM gene carriers being estimated at 1:200.4 In about 60% of cases, transmission of the disease is autosomal dominant, caused by mutations in cardiac sarcomere protein genes.5
Dynamic left ventricular outflow tract (LVOT) obstruction is a hallmark of the disease, caused by systolic anterior motion (SAM) of the anterior mitral leaflet.6 The presence of LVOT obstruction, defined as a resting systolic gradient of >30 mmHg in the LVOT, has been associated with worse outcome, and can lead to heart failure, arrhythmic events, stroke and death.2,7–9 Studies have shown that reduction of LVOT obstruction by surgery or percutaneous alcohol septal ablation (ASA) improves prognosis.10–12
According to the current guidelines, symptomatic patients in New York Heart Association (NYHA) class ≥III and/or with recurrent exertional syncope, refractory to medical management and associated with an outflow gradient ≥50 mmHg, are candidates for septal reduction intervention (class I recommendation, level of evidence B).2 The decision between a surgical or a percutaneous approach depends on mitral valve and septal anatomy and the presence of other lesions requiring surgical intervention.
The aim of the present study was to assess the efficacy and safety of ASA and its long-term outcome.
MethodsStudy designWe analyzed consecutive patients who underwent ASA in a reference center. The data were collected prospectively between beginning the procedure in January 2009 and June 2017.
Selected patients presented NYHA class III or IV, Canadian Cardiovascular Society (CCS) class II or III angina, or exertional syncope for which an arrhythmic cause had been excluded. Dynamic obstruction was defined as ≥50 mmHg at rest and/or ≥70 mmHg with provocation. Minimum basal septal thickness was >16 mm, given the risk of causing an iatrogenic interventricular communication. Significant functional mitral regurgitation due to SAM was not considered a contraindication for ASA, but in cases of concurrent regurgitation mechanism myectomy should be performed. In the presence of severe hypertrophy (>30 mm), mid-ventricular gradient and LVOT obstruction caused by the papillary muscle, surgical myectomy was the procedure of choice. The patients who underwent ASA in our sample were considered at high risk for surgery or expressed their personal preference for ASA.
Baseline clinical characteristics including demographic characteristics, heart failure symptoms, presence of angina and comorbidities were collected for all patients.
Major complications were defined as those resulting in death or significant morbidity or life-threatening events during the procedure or follow-up.13 Heart failure was classified according to NYHA functional class.
All patients were followed periodically. In addition to outpatient clinical visits, telephone follow-up was performed to check for the presence of symptoms and the occurrence of cardiac events.
In the majority of patients a dedicated echocardiogram was performed at three months and/or within 12 months of the procedure. End-diastolic interventricular septal thickness was assessed by M-mode and 2D echocardiography in parasternal long-axis view and 4 chambers. LVOT gradient at rest and after provocation with Valsalva maneuver was assessed by a combination of color Doppler, pulsed wave Doppler and continuous wave Doppler echocardiography. Mitral regurgitation was assessed by color Doppler and in some cases by the proximal isovelocity surface area method.
The procedure was defined as successful when a >50% reduction in LVOT gradient was achieved at one-year follow-up.
Study endpointsPrimary endpointsThe primary echocardiographic endpoint was >50% reduction in LVOT gradient within a year of the procedure.
The primary clinical endpoints were improvement in functional capacity and a combined endpoint of cardiac death and rehospitalization for cardiac cause.
The primary safety endpoint was a combination of in-hospital mortality and major complications.
Secondary endpointsThe secondary echocardiographic endpoint was improvement in mitral regurgitation, and the secondary clinical endpoint was a combination of all-cause mortality and rehospitalization.
Alcohol septal ablationASA was performed in a standard fashion as previously described.13,14 The septal branch of the left coronary artery was chosen by invasive angiography. An angioplasty balloon was inflated in the proximal portion of the septal branch and 1-2 ml of SonoVue® echocardiographic contrast (Bracco, Milan, Italy) was injected through the balloon lumen. The selected artery was assessed by contrast echocardiography as a suitable target for ASA. If the branch constituted a good choice, 0.1 ml alcohol per cm thickness was infused at a rate of 1 ml/min.
The risk of need for permanent pacemaker was estimated using the criteria described by Faber et al.15
Statistical analysisThe statistical analysis was performed using IBM SPSS Statistics (IBM SPSS, Chicago, IL). Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as absolute number and percentage. Normality was assessed using the Shapiro-Wilk test. Study groups (successful vs. unsuccessful) were compared using the Student's t test or the Wilcoxon-Mann-Whitney test for continuous variables, and Pearson's chi-square or Fisher's exact test for categorical measures, as appropriate. A p-value <0.05 was considered statistically significant.
ResultsDuring the study period, ASA was performed in 80 patients, mean age 63.9±12.3 years, 30.0% male. The baseline clinical characteristics of the study population are presented in Table 1.
Baseline clinical characteristics of the overall population.
| Age, years | 63.9±12.3 |
| Male, n (%) | 24 (30.0) |
| NYHA class III/IV, n (%) | 74 (92.5) |
| CCS class II/III, n (%) | 42 (52.5) |
| Syncope, n (%) | 6 (7.5) |
| Hypertension, n (%) | 47 (58.8) |
| Diabetes, n (%) | 6 (7.5) |
| Dyslipidemia, n (%) | 34 (42.5) |
| Coronary disease, n (%) | 7 (8.8) |
| Atrial fibrillation, n (%) | 14 (17.5) |
| Previous PPM, n (%) | 10 (12.5) |
| Maximum septal thickness, mm | 21.6±3.1 |
| LVOT gradient, mmHg | 96.3±34.6 |
| Moderate MR, n (%) | 26 (32.5) |
| SAM, n (%) | 36 (45.0) |
| LV diameter, mm | 45.6±8.1 |
| LA diameter, mm | 47.8±8.9 |
| PASP, mmHg | 46.3±18.1 |
| Beta-blocker, n (%) | 63 (78.8) |
| CCB, n (%) | 34 (42.5) |
CCB: calcium channel blocker; CCS: Canadian Cardiovascular Society; LA: left atrial; LV: left ventricular; LVOT: left ventricular outflow tract; MR: mitral regurgitation; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; PPM: permanent pacemaker; SAM: systolic anterior motion of anterior mitral leaflet.
Regarding clinical features, most patients (92.5%) presented NYHA class III or IV heart failure, around half (52.5%) presented CCS class II or III angina, and only 7.5% presented syncope.
As shown in Table 1, mean LVOT gradient at baseline was 96.3±34.6 mmHg and maximum septal thickness was 21.6±3.1 mm. Regarding the mitral valve, 45.0% of patients presented SAM and 32.5% presented moderate mitral regurgitation (≥II/IV).
Reflecting septal thickness, the mean alcohol dose was 2.04±0.36 ml. Mean peak creatine kinase was 1233.6±591.2 U/l.
Six minor complications were observed in 6.3% of patients (n=5), including infection in two patients resolved by antibiotic therapy, rapid atrial fibrillation in one patient controlled with pharmacological therapy, acute pulmonary edema in one patient resolved with medical management only, and vascular access site complications in two patients without need for surgical intervention.
Major complications were observed in 2.5% of patients (n=2), consisting of one case of electrocatheter-related cardiac tamponade resolved by pericardiocentesis, and one case of inferior infarction caused by recruitment of the collaterals of the target vessel to the posterior interventricular branch.
Atrioventricular block was observed in 45.0% of patients (n=36), in the majority of cases transiently during ASA and/or within 12 hours of the procedure. Only 8.8% of patients received a permanent pacemaker for atrioventricular block, of whom two received an implantable cardioverter-defibrillator (ICD) due to non-sustained ventricular tachycardia previously documented on Holter monitoring, and another patient due to risk of cardiac sudden death of 6% estimated by a validated scoring system.2
Redo ASA was performed in eight patients (10.0%) and surgical myectomy in two patients (2.5%) due to recurrence of LVOT gradient and symptoms and lack of a target septal branch, respectively.
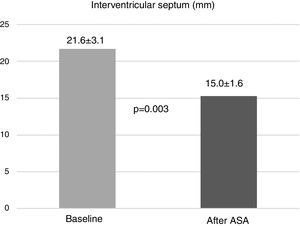
Significant reductions in mean septal thickness were seen, from 21.6±3.1 mm at baseline to 15.0±1.6 mm on echocardiographic assessment three months after ASA (p=0.003) (Figure 1).
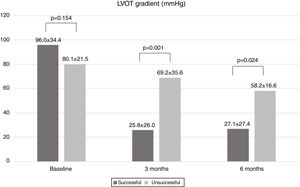
Subsequent echocardiographic parameters after ASA were missing in three patients who were followed at different centers. The primary echocardiographic endpoint was achieved by 66 patients (85.7%). At three-month follow-up, LVOT gradient in the overall population was 30.2±31.3 mmHg. As shown in Figure 2, mean LVOT gradient was significantly lower in the successful group (25.8±26.0 mmHg compared to 69.2±35.6 mmHg in the unsuccessful group; p=0.001). At six months, mean LVOT gradient was 33.1±28.3 mmHg in the overall population, substantially lower in the successful group (27.1±27.4 mmHg vs. 58.2±16.6 mmHg; p=0.024) (Figure 2). In terms of baseline characteristics, there were no significant differences between patients who achieved and who did not achieve the primary echocardiographic endpoint. There were also no significant differences in alcohol administration or peak creatine kinase between the two groups (Table 2).
Comparison between successful and unsuccessful groups.
| Characteristics | Successful (n=66) | Unsuccessful (n=11) | p |
|---|---|---|---|
| Age, years | 64.9±11.5 | 56.5±16.1 | 0.173 |
| Male, % | 33.3 | 18.2 | 0.265 |
| Hypertension, % | 62.3 | 54.5 | 0.684 |
| Diabetes, % | 6.3 | 18.2 | 0.377 |
| Dyslipidemia, % | 45.3 | 45.5 | 0.835 |
| Dyspnea, % | 90.8 | 100 | 0.576 |
| NYHA class III, % | 87.5 | 90.9 | 0.607 |
| NYHA class IV, % | 7.8 | 0 | 0.442 |
| Angina, % | 53.8 | 45.5 | 0.780 |
| Baseline septal thickness, mm | 21.3±2.9 | 23.4±4.4 | 0.490 |
| Baseline LVOT gradient, mmHg | 96.0±34.4 | 80.1±21.5 | 0.154 |
| SAM, % | 50.0 | 18.1 | 0.050 |
| Moderate MR, % | 34.8 | 27.3 | 0.623 |
| LV diameter, mm | 46.3±8.7 | 41.7±6.8 | 0.206 |
| LA diameter, mm | 47.8±8.9 | 47.8±9.6 | 0.991 |
| PASP, mmHg | 47.0±18.6 | 37.5±3.6 | 0.488 |
| Beta-blocker, % | 78.5 | 81.8 | 1.000 |
| CCB, % | 47.7 | 27.3 | 0.327 |
| Sinus rhythm, % | 87.5 | 90.0 | 1.000 |
| Alcohol, ml | 2.07±0.27 | 2.04±0.33 | 0.747 |
| Peak creatine kinase, U/l | 1233.1±600.3 | 1106.3±555.5 | 0.551 |
CCB: calcium channel blocker; LA: left atrial; LV: left ventricular; LVOT: left ventricular outflow tract; MR: mitral regurgitation; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; SAM: systolic anterior motion of the anterior mitral leaflet.
Success was defined as achievement of the primary echocardiographic endpoint.
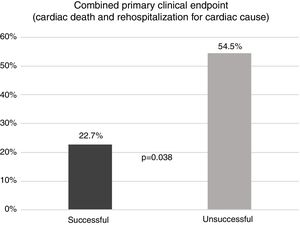
Regarding the primary clinical endpoints, improvement in functional capacity was achieved in the majority of patients. Of the 74 patients in NYHA class III/IV before the procedure, 57 (77%) improved to NHYA class I/II. The combined primary clinical endpoint of cardiac death and rehospitalization for cardiac cause was observed in 27.5% of patients in the overall population (n=22) during a follow-up of 3.9±2.3 years. In patients who did not present LVOT gradient reduction of >50% (the criterion for procedural success), the combined primary endpoint was observed in more than half (54.5%), compared to only 22.7% of patients with procedural success (p=0.038) (Figure 3). Only two patients (2.7%) died of cardiac causes, one from septic shock after surgical myectomy 55 months after ASA and the other from cardiogenic and septic shock during hospitalization for ASA, 15 days after the procedure.
The safety endpoint was observed in three patients (3.8%), including two major complications and one in-hospital death, as described above.
Concerning the secondary endpoints, among the 26 patients with moderate mitral regurgitation at baseline, 50% improved to mild regurgitation, as assessed by follow-up echocardiography.
The secondary clinical endpoint (all-cause mortality and rehospitalization) was observed in 40.0% of the patients (n=32), with four deaths of non-cardiac cause (two from cancer, one from sepsis and one from pulmonary embolism). Two of the non-cardiac deaths occurred within a year of ASA (11 and 12 months after the procedure).
During follow-up one patient received an ICD due to ventricular fibrillation induced in electrophysiological study.
In the four patients with ICDs (one implanted before ASA), no sustained ventricular arrhythmic events were documented by the device and only one patient presented non-sustained ventricular tachycardia. In the other patients, non-sustained ventricular tachycardia was documented on Holter monitoring in 13 patients (16.3%) and ventricular triplets in five (6.3%). No sustained ventricular tachycardia was documented on Holter recordings.
Results on the efficacy and safety of the procedure are summarized in Table 3.
Efficacy and safety of alcohol septal ablation.
| Efficacy | |
| Primary echocardiographic endpoint: >50% reduction in LVOT gradient during 1st year | 85.7% |
| Primary clinical endpoints | |
| Improvement in functional capacitya | 77.0% |
| Combined endpoint: cardiac death and rehospitalization for cardiac causeb | 27.5% |
| Secondary echocardiographic endpoint: improvement in mitral regurgitationc | 50% |
| Secondary clinical endpoint: all-cause mortality and rehospitalizationb | 40.0% |
| Safety | |
| Primary safety endpoint: in-hospital mortality and major complication | 3.8% |
| Major complications | 2.5% |
| Minor complications | 6.3% |
| Atrioventricular block (transient or permanent) | 45.0% |
| Permanent pacemaker implantation | 8.8% |
| Redo ASA/surgical myectomy | 12.5% |
ASA: alcohol septal ablation; LVOT: left ventricular outflow tract; NYHA: New York Heart Association.
The present article demonstrates the efficacy and safety of ASA, as well as its benefit in short- and long-term outcomes.
Successful ASA, defined by a primary echocardiographic endpoint of a >50% LVOT gradient reduction within a year of the procedure, was achieved in 85.7% of our population, with residual LVOT gradients of 20-30 mmHg, consistent with published studies.16–19
ASA reduces gradients by increasing LVOT area, resulting in symptomatic relief, improvement of systolic and diastolic function, reduction of mitral regurgitation and improvement of coronary flow reserve.18,20–24 The reduction in LVOT gradient leads to a decrease in afterload and consequent increase in cardiac output. As a result of these hemodynamic changes, diastolic pressure in the aorta increases and diastolic pressure in the LV chamber decreases, resulting in higher coronary filling pressure and better myocardial perfusion, improving LV performance. Regarding diastolic function, previous studies based on assessment by pressure-volume loops or echocardiography showed improvement in myocardial relaxation properties, particularly active relaxation, leading to better diastolic compliance and distensibility.18,25,26
It should be borne in mind that the maximum beneficial effect of LVOT gradient reduction is achieved when final remodeling and scarring occur, usually more than three months after the procedure.27 Echocardiographic assessment during the first year of follow-up, rather than only in the first few months, is therefore essential to determine definitive procedural success.28 Yoeger et al. described a ‘triphasic’ gradient response, consisting of an early reduction immediately after ASA as a result of decreased contractility of stunned myocardium after ischemia, followed by a rebound in gradient when contractility recovers, and the final reduction after three months, as described above.27
In line with previous studies, we also achieved significant reductions in NHYA functional class and in severity of mitral regurgitation in the majority of patients.19,24,28 Baseline interventricular septal diameter was significantly reduced after ASA, as demonstrated by follow-up echocardiograms, as expected according to published experiences.19,29
Although we found no significant differences in baseline characteristics between successful and unsuccessful groups, previous studies identified larger quantities of injected alcohol, higher baseline LV ejection fraction and LVOT outflow gradient, post-procedural bundle branch block on ECG, and older age as independent predictors of significant reduction in LVOT gradient.19
According to the current European guidelines on HCM, ASA may be less effective in the presence of very severe hypertrophy (≥30 mm) and extensive septal scarring.2 In cases of mid-cavity obstruction or a secondary cause of mitral regurgitation apart from SAM, surgical myectomy should be the procedure of choice.
Regarding safety, high-degree atrioventricular block is the most frequent complication. Its incidence has decreased with the use of contrast echocardiography to guide alcohol injection and identify the target area at risk, and the use of smaller quantities of alcohol. In our experience, most cases were transient, presumably due to perinecrotic inflammation, and only 8.8% of cases needed a permanent pacemaker due to permanent conduction tissue damage. Our results are in agreement with previous series that observed a pacemaker implantation rate of less than 10%.16,17,19
A study with contrast-enhanced cardiac magnetic resonance imaging confirmed that infarct size correlated with alcohol volume and electrocardiographic changes with infarct location.30
Procedure-related complications included one case of cardiac tamponade, one of inferior myocardial infarction and two of vascular access site complications without need for surgical intervention. Cardiac tamponade has been reported as a complication of ASA in previous series.19,31 There was only one in-hospital death, 16 days after ASA, not secondary to a procedure-related complication. This patient was in NYHA class IV with mid-ventricular obstruction, and the procedure was a palliative intervention. These findings confirm the low mortality associated with this procedure reported in the literature, even in high-risk patients.32,33 In a long follow-up period, we reported all-cause mortality of 7.2% and mortality of cardiac cause of 2.5%. In our series there was only one death (1.3%) in the first 30 days after ASA, in line with a systematic review by Alam et al. which reported mean 30-day mortality of 1.5% (0.0-5.0%).34
Despite concerns about ventricular arrhythmias secondary to a permanent arrhythmogenic substrate generated by ASA scarring, published studies have not shown increased incidence of arrhythmic events after ASA. In our study no sustained ventricular arrhythmic events were recorded during follow-up.
During a long follow-up (mean of 3.9±2.3 years since beginning the procedure in January 2009), cardiac events including hospitalization and death occurred mainly in patients in whom significant reduction of LVOT gradient was not achieved. The mean annual cardiac mortality of 0.5% and annual all-cause mortality of 1.0% in our study were slightly less than in the series by Steggerda et al. (0.7% and 1.5%, respectively).31
With regard to efficacy and safety, ASA can now be considered as an alternative to surgery, presenting the advantage of being a percutaneous procedure that does not require sternotomy and is therefore associated with shorter recovery times. The technique can also be used in patients with contraindications for surgery due to age or comorbidities. However, when mitral valve repair is needed and in cases requiring a larger quantity of hypertrophied muscle to be resected, surgery should be the first option. The long-term outcomes are similar between the two approaches in functional class, LVOT gradient reduction, mitral abnormalities and ventricular arrhythmias.3,34 With ASA, periprocedural complications are less frequent and hospital stay is shorter.31 Nevertheless, there is more frequently need for permanent pacemaker implantation with ASA, and immediately after the procedure LVOT gradients are higher with this technique.31,34,35
Our study has some limitations. The study sample size inevitably reflects the limitations of a single-center experience. However, given the relatively small number of patients who have undergone this technique, a larger study population would prove difficult to achieve, except in multicenter studies. Referral for ASA was based on therapeutic decisions made by referring physicians, and it is likely that some patients with formal indication were not referred. As our department is a reference center, some patients are followed in other centers, and consequently in these patients follow-up data were obtained only by telephone, which can lead to errors in classification of functional capacity and recording of cardiac events and complications. Furthermore, the study did not include a control group to compare the outcomes in a similar population that did not undergo ASA.
ConclusionASA has a high success rate in patients with obstructive HCM, with significant decreases in LVOT gradient, improvement of functional capacity and/or reduction of mitral regurgitation in most cases. Patients who achieve significant reductions in LVOT gradient suffered less cardiac death and cardiac rehospitalization for cardiac cause.
ASA is also a safe procedure, most cases of high-degree atrioventricular block being transient, with a rate of permanent pacemaker implantation below 10%.
Considering its efficacy and safety profile, ASA can now be considered as an alternative to surgery in patients with obstructive HCM.
Conflicts of interestThe authors have no conflicts of interest to declare.