Over 60 years ago, Donald Teare1 described eight cases of young patients, seven of whom died suddenly, whose hearts presented asymmetric septal hypertrophy resembling a cardiac tumor, which he termed “a muscular hamartoma of the heart”. Since that time much has changed in our knowledge of hypertrophic cardiomyopathy (HCM).
With regard to phenotypes, unlike what was previously believed, the hallmark of the disease (septal hypertrophy) is only seen in about two-thirds of patients.2 Even fibrosis can now be quantified by cardiac magnetic resonance imaging.3
Information on hemodynamics in HCM has advanced from the first descriptions of abnormal systolic anterior motion (SAM) of the mitral valve and resulting mitral regurgitation on angiography in the mid-1960s4 to the ability to quantify obstruction at rest by provocative maneuvers or exercise testing.5
The view of the importance of genomics in HCM has also changed, moving toward broader, more inclusive models that include both biological risk and acquired factors to explain the disease. Mutations in a single sarcomere gene are no longer considered an adequate driving force for the disease, since they do not properly explain regional left ventricular hypertrophy and myocardial fibrosis, as well as structurally abnormal elongated mitral valve leaflets and remodeled intramural coronary arterioles, which involve tissue types that do not express cardiomyocyte sarcomere proteins.6
In terms of outcomes, in contrast to what was initially thought, most individuals with HCM have near-normal life expectancy, and many remain asymptomatic throughout life, only some patients develop heart failure, angina, syncope or even sudden cardiac death, which may be caused by different mechanisms.7
There is nowadays a range of treatments available for HCM, including behavior modification, devices, drugs, and surgical or percutaneous intervention.7,8
Nearly two-thirds of patients with HCM have a significant gradient across the left ventricular outflow tract (LVOT) at rest or during provocative maneuvers or exercise. LVOT obstruction results from the combined effects of septal hypertrophy and abnormalities of the mitral valve apparatus (systolic flow drags the elongated and abnormally positioned anterior mitral leaflet into the LVOT). Coaptation of the mitral leaflet is distorted, resulting in dynamic mitral regurgitation, which plays an important role in symptoms. LVOT obstruction has several pathophysiological consequences, including reduction of cardiac output, diastolic dysfunction, secondary mitral regurgitation, and myocardial ischemia.9 A resting LVOT gradient of ≥30 mmHg is a predictor of both all-cause mortality and arrhythmic events.7,8
The main treatment in these patients is negative inotropic drugs (beta-blockers, calcium channel blockers and disopyramide), but 5-10% remain symptomatic and need additional therapy, such as pacemaker implantation, surgical septal myectomy or alcohol septal ablation (ASA).10
The 2011 AHA/ACC guidelines8 consider septal myectomy the gold standard technique for septal reduction therapy, and advise against performing ASA in patients who are younger or who present marked septal hypertrophy (>30 mm) or concomitant cardiac disease. ASA can be offered to elderly patients, those with high surgical risk, and those who refuse open-heart surgery. As there have been no randomized trials comparing surgery and ASA, the guidelines are based on observational studies, but current evidence11 is closer to the European guidelines, which accept both approaches, and recommend individual assessment based on a heart team discussion.
Three meta-analyses – Agarwal et al.12 (2010), Leonardi et al.13 (2010), and Liebregts et al.14 (2015) – have confirmed that both procedures are efficacious, with no differences in symptom relief, safety, or mortality, only a more frequent need for pacemaker implantation with ASA. The fear that additional scar tissue secondary to ablation could result in an arrhythmic substrate, sudden cardiac death or evolution to systolic dysfunction is now a thing of the past.15,16
Regarding treatment with ASA of LVOT gradients refractory to medical therapy, the study by Rosa et al.17 published in this issue of the Journal adds robustness to previous data. The authors report a mean 50% reduction in LVOT gradient within a year of the procedure in 85.7% of patients, improved New York Heart Association functional class in 77%, permanent pacemaker implantation in 8.8%, redo ASA in 10%, myectomy in 2.5%, and cardiac death in 2.7% (two patients), similar figures to the Euro-ASA registry19 and high-volume centers (Table 1).
Comparison between the results of Rosa et al.17 and previous data on alcohol septal ablation.
| No. of patients | Improved symptoms | Pacemaker implantation | In-hospital cardiovascular mortality | Total cardiovascular mortality | |
|---|---|---|---|---|---|
| Rosa et al.17 (2019) | 80 | 77% | 8.8% | 1.25% | 2.5% |
| Batzner et al.18 (2019) | 952 | 94.3% | 10.5% | 0.21% | 1.47% |
| US Nationwide Inpatient Database11 (2016) | 4862 (248 centers) | - | Total: 11.9%1st T: 14.2%2nd T: 12.4%3rd T: 11.5% | Total: 0.7%1st T: 0.3%2nd T: 0.8%3rd T: 0.6% | - |
| Euro-ASA Registry19 (2016) | 1275 (10 centers) | 86% | 12% | 1% | 2.4% |
T: tertile of hospital volume.
Concerns about differences in outcomes between high- and low-volume centers have been voiced on both sides of the Atlantic. In the US Nationwide Inpatient Database,11 myectomy had different outcomes in high- and low-volume centers, whereas this was not seen with ASA. Veselka at al.20 showed that in the Euro-ASA Registry, the first consecutive 50 patients treated in each center had worse outcomes than patients treated thereafter. Although Rosa et al.17 only performed around 10 ablations per year (80 patients in seven years), their excellent results provide reassurance that ASA can be a viable option for patients in Portugal to relieve obstruction without compromising safety even in relatively young patients (mean age 63.9±12.3 years). The safety of the procedure even in low-volume centers can be explained by the high degree of skill required to treat coronary total occlusion percutaneously, which interventional cardiologists who perform a large number of angioplasties achieve on a daily basis. The major challenges facing those who perform ASA are firstly patient selection (anatomy, gradient, previous medical therapy, comorbidities), secondly how much alcohol to infuse and into which septal branch(es), and lastly management of the cardiac conduction system. Unfortunately Rosa et al. could not add any data to help optimize patient selection, mainly because they were unable to find any markers that were correlated with success.
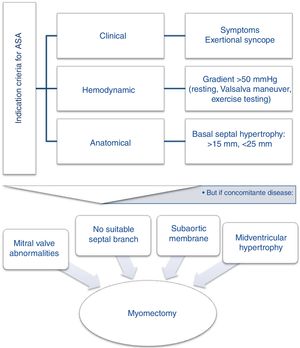
Since randomized trials comparing ASA and surgical myectomy are unlikely to occur, the report by Rosa at al. supports the latest consensus21,22 that, if deemed suitable23 after a heart team discussion (Figure 1), both techniques should be proposed to patients, explaining the advantages and disadvantages of each, and taking the patient's wishes into account.
Current evidence based on the latest knowledge, such as that provided in the report by Rosa et al. and others, sheds light on the options available for treating HCM, and should motivate physicians to fight inertia and remember in their daily practice that their patients could be relieved by alcohol.
Conflicts of interestThe author has no conflicts of interest to declare.