Transcatheter aortic valve implantation (TAVI) has become an important treatment in high surgical risk patients with severe aortic stenosis (AS), whose complications need to be managed promptly.
The authors report the case of an 86-year-old woman presenting with severe symptomatic AS, rejected for surgery due to advanced age and comorbidities. The patient underwent a first TAVI, with implantation of a Medtronic CoreValve®, which became dislodged and migrated to the ascending aorta. Due to the previous balloon valvuloplasty, the patient's AS became moderate, and her symptoms improved. After several months, she required another intervention, performed with a St. Jude Portico® repositionable self-expanding transcatheter aortic valve. There was a good clinical response that was maintained at one-year follow-up.
The use of a self-expanding transcatheter bioprosthesis with repositioning features is a solution in cases of valve dislocation to avoid suboptimal positioning of a second implant, especially when the two valves have to be positioned overlapping or partially overlapping each other.
A implantação de válvula aórtica percutânea (VAP) tornou-se um procedimento importante no tratamento de doentes com estenose aórtica grave com elevado risco cirúrgico, cujas complicações devem ser avaliadas e tratadas de forma adequada.
Os autores relatam o caso de uma doente de 86 anos, com estenose aórtica grave sintomática, recusada para intervenção cirúrgica dada a idade avançada e comorbilidades. A doente foi submetida a uma primeira implantação de VAP com uma válvula Medtronic CoreValve®, a qual sofreu um deslocamento para a aorta ascendente durante o procedimento. Devido à angioplastia de balão previamente efetuada, a estenose aórtica tornou-se moderada, com melhoria da sintomatologia da doente. Vários meses depois, por agravamento clínico, houve necessidade de reintervenção, a qual foi realizada com um sistema reposicionável Portico®. Verificou-se um segundo posicionamento valvular adequado, com boa resposta clínica da doente, que persiste após um ano de follow-up.
A utilização de sistemas reposicionáveis de implantação de VAP constitui, atualmente, uma solução em casos de deslocamento da válvula, de forma a evitar uma segunda implantação subótima, sobretudo quando as duas válvulas têm de ficar em sobreposição.
Transcatheter aortic valve implantation (TAVI) has become an alternative to surgical aortic valve replacement (SAVR) in high-risk patients with severe aortic stenosis (AS).1,2 The complications associated with this procedure are different from those related to SAVR. This article describes the case of a patient who suffered dislocation of a Medtronic CoreValve® during initial TAVI treatment. Eventually, after several months of moderate improvement, she needed another intervention, which was performed with a St. Jude Portico® repositionable self-expanding transcatheter aortic valve.
Case reportThe authors report a case of an 86-year-old woman presenting with New York Heart Association (NYHA) class II symptoms and severe AS (maximum and mean transvalvular gradients of 199 and 75 mmHg, respectively; aortic valve area 0.7 cm2). She had preserved left ventricular systolic function and non-significant coronary artery disease, and was rejected for SAVR due to advanced age, low weight and extreme frailty (logistic EuroSCORE I and II 19.79% and 4.22%, respectively; Society of Thoracic Surgeons score 3.7%).
The patient underwent a first TAVI in 2012, through angiography-guided femoral access. Valvuloplasty was performed with a 20 mm NuMED Nucleus balloon followed by implantation of a 26 mm CoreValve®. During this procedure, the valve became dislodged and migrated to the ascending aorta due to excessive tension on the first-generation delivery catheter making it uncontrollable during fine manipulation of the valve release. A snare technique was used to reposition the prosthesis upward, without affecting the coronaries. Given the fact that valvuloplasty had been performed and the percutaneous aortic valve was of the first generation and not repositionable, it was decided to suspend the procedure and postpone any possible second intervention. The patient's AS became moderate, with maximum and mean gradients decreasing to 67 and 35 mmHg, respectively, and she improved to NYHA class I. No neurologic, aortic or peripheral vascular complications occurred.
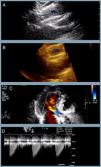
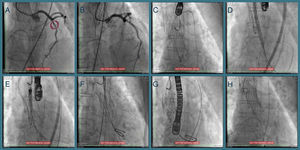
One year later her AS worsened (maximum and mean transvalvular gradients of 101 and 61 mmHg, respectively; aortic valve area 0.8 cm2) and, in 2014, she was hospitalized for congestive heart failure. The heart team decided on a new TAVI with a repositionable system as the first-line approach, and a balloon-expandable TAVI as a second-line solution. Percutaneous coronary intervention (due to progression of coronary artery disease) was followed by transfemoral TAVI (valve-in-valve) under three-dimensional transesophageal echocardiographic guidance (Figure 1). The CoreValve® was immobilized with a 15 mm snare loop by a radial approach. After valvuloplasty with a 20 mm Nucleus balloon, a 25 mm Portico® valve was implanted. Post-dilation was performed for underexpansion. The patient was prescribed dual antiplatelet therapy with aspirin and clopidogrel until six months after the second procedure.
Sequential procedures during the second intervention. (A and B) Percutaneous coronary intervention of the mid left anterior descending artery with a drug-eluting stent; (C) transfemoral transcatheter aortic valve implantation (valve-in-valve) under three-dimensional transesophageal echocardiographic guidance, the Medtronic CoreValve® being immobilized with a 15 mm snare loop by a radial approach; (D) aortic valve balloon valvuloplasty performed with a 20 mm NuMED Nucleus balloon, followed by (E and F) implantation of a 25 mm St. Jude Portico® valve; (G) this valve was underexpanded and post-dilation was performed after changing the Amplatz super stiff wire for a backup Meier wire in order to advance the Nucleus balloon; (H) final result.
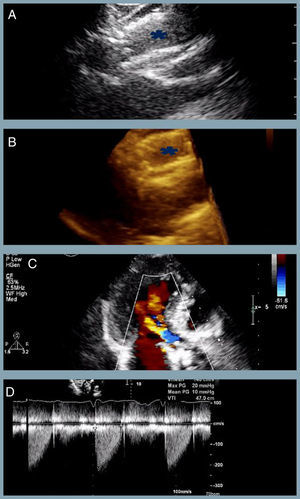
At one-year follow-up the patient remains in NHYA class II, with NT-proBNP level of 510 pg/ml. Transthoracic echocardiography revealed the two bioprostheses overlapping with appropriate transprosthetic gradients and associated mild to moderate leak (Figure 2).
The last transthoracic echocardiography (at one-year follow-up) revealing (A and B) partial overlapping of the two bioprostheses (blue asterisk) (two- and three-dimensional respectively); (C) mild to moderate associated periprosthetic leak; (D) appropriate transprosthetic gradients (maximum and mean of 20 mmHg and 10 mmHg, respectively).
Malpositioning of the bioprosthesis during TAVI is a serious procedural complication which needs to be managed promptly to avoid vascular and other systemic complications. Two prospective studies conducted in patients undergoing TAVI using the CoreValve® revealed a prevalence of valve dislocation after deployment of 3.2-3.9%, with no long-term vascular or neurological complications.1,3 Another study observed this complication in about 10% of patients, associated with lower survival rates and higher frequency of coronary ischemia, stroke, and renal failure.2
Possible causes of malpositioning are mismatch of the annulus and valve size, which can be solved by precise annulus measurements with echocardiographic and computed tomography imaging; arrhythmias, which hinder stabilization of the valve during deployment (higher ventricular rate pacing is used to stabilize the valve); and by accident, after successful deployment during retraction of the delivery system, if the anchors are not fully released from the deployment catheter or if the tip of the deployment catheter becomes caught at the proximal end of the prosthesis.2
Besides morphological or patient-related factors, operator experience appears to be crucial to the prevalence of valve dislocation.1,2 This was confirmed in a reference center, in which the incidence of CoreValve® dislocation decreased constantly with increasing experience over a period of almost three years.2
Valve dislocation after complete deployment often results in the need to implant a second prosthesis, which is a feasible and effective interventional option that appears to be safer than retrieving the first prosthesis.1 The decision whether to insert a new valve inside the previous one, or to pull the first one to another location in the aorta and implant a second valve sequentially, depends on various factors, including operator preferences and procedural circumstances.3 When the two valves are positioned overlapping or partially overlapping each other, there are various disadvantages: duplication of the amount of metal residing against the native aortic wall increases the risk of covering the native coronary ostia and requires more time for endothelialization.3 Emergent surgery may be necessary in some cases.2
New transcatheter heart valves have been created in order to overcome some of the limitations of previous systems.4 The Portico® is a self-expanding transcatheter aortic bioprosthesis with novel capabilities.4 Its ability to be repositioned, recaptured, and redeployed is a desirable feature, especially when the initial implant positioning is suboptimal. However, only small series with limited follow-up have been published.4 Only one case of successful Portico® transcatheter aortic valve-in-valve implantation, in a patient with a degenerated bioprosthesis (placed surgically), has been described to our knowledge.5 This is the first reported case of TAVI valve-in-valve with this type of bioprosthesis, with good results at 12 months. A valve-in-valve procedure with a self-expanding transcatheter bioprosthesis with repositioning features is presently the first-line solution in cases of degenerated biological prostheses or prosthesis displacement during TAVI. However, assessment of more patients in medium- and long-term follow-up is required.
ConclusionsDislocation of the bioprosthesis during TAVI is a serious procedural complication which needs to be managed promptly to avoid vascular and other systemic complications. This report describes a successful valve-in-valve procedure after valve dislodgement, with implantation of a self-expanding transcatheter bioprosthesis with repositioning capabilities.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Maria Conceição Furstenau (Serviço de Anestesiologia, Hospital Santa Cruz, Carnaxide, Portugal), Regina Ribeiras and Miguel Mendes (Serviço de Cardiologia, Hospital Santa Cruz, Carnaxide, Portugal), and Ana Aleixo (Centro de Estudos de Doenças Crónicas, Faculdade de Ciências Médicas, Lisboa, Portugal).