Pulmonary vein (PV) isolation is one of the cornerstones of rhythm-control therapy for symptomatic atrial fibrillation (AF) patients. Pulsed field ablation (PFA) is a novel ablation modality that involves the application of electrical pulses causing cellular death, and it has preferential tissue specificity. In this study, we aimed to share a one-year single center experience of AF ablation with PFA.
MethodsSingle center, retrospective study of consecutive patients undergoing PVI using the pentaspline PFA catheter between June 2022 and July 2023. Data on demographic, procedural, and electrocardiographic recurrence (assessed after a three-month blanking period) were analyzed.
ResultsOne hundred twenty-three consecutive patients were included (62±11 years, 59% male), with a mean CHA2DS2-VASc score of 2±1 points, median left ventricular ejection fraction of 61% [IQR 60–65%] and a median left atrial volume index (by CT scan) of 55 mL/m2 [IQR 41–67 mL/m2]. Fifty-two percent of patients presented paroxysmal AF and 21 patients (17%) underwent a redo ablation. Median procedure time was 83 min [IQR 59–117 min] and median fluoroscopy time was 11.6 min [IQR 8.2–15.6 min]; posterior wall isolation was performed in 43 (35%). Two patients (1.6%) experienced acute cardiac tamponade, immediately treated with pericardiocentesis. Other complications were primarily vascular, in 4% of cases (three femoral hematomas, one femoral pseudoaneurysms, one arteriovenous fistula). Over 290 (IQR 169–387) days of follow-up, considering electrocardiographic recurrence beyond the blanking period, 9% of patients had AF recurrence (two with paroxysmal AF and nine with persistent AF).
ConclusionsPulsed field ablation for PVI and posterior wall ablation was an efficient and safe procedure with low rate of complications and high percentage of patients were free from AF in short-term follow-up. We need more studies to evaluate long-term success.
O isolamento das veias pulmonares (IVP) é um dos pilares da terapêutica de controlo de ritmo em doentes com fibrilhação auricular (FA) sintomática. A ablação por eletroporação/pulse field ablation (PFA) é uma nova modalidade de ablação que causa a morte celular através da aplicação de pulsos elétricos com especificidade para o tecido miocárdico. Neste estudo, o nosso objetivo é partilhar a experiência de ablação de FA com PFA, num ano, num centro único.
MétodosEstudo retrospetivo de centro único, que inclui doentes submetidos a IVP com PFA entre junho de 2022 e julho de 2023. Foram colhidos e analisados dados demográficos, dados relativos ao procedimento, bem como à recidiva eletrocardiográfica (avaliada após o período blanking).
ResultadosForam incluídos 123 doentes (62±11 anos, 59% homens), com score CHA2DS2-VASc médio de 2±1 pontos, fração de ejeção ventricular esquerda mediana de 61% [IQR 60-65%] e volume auricular esquerdo mediano (por tomografia computadorizada) de 55mL/m2 [IQR 41-67mL/m2]. 52% dos doentes tinham FA paroxística e 21 doentes (17%) estavam a realizar um procedimento de re-ablação de FA. O tempo médio do procedimento foi de 83min [IQR 59-117min] e o tempo médio de fluoroscopia foi de 11,6min [IQR 8,2–15,6min]; 43 doentes (35%) realizaram isolamento da parede posterior. Dois doentes (1,6%) tiveram tamponamento cardíaco, tratado prontamente com pericardiocentese. 4% dos doentes tiveram uma complicação vascular (3 hematomas femorais, 1 pseudoaneurisma femoral, 1 fístula arteriovenosa). Num follow-up mediano de 290 dias (IQR 169–387), considerando recorrência eletrocardiográfica, além do período de blanking, 9% dos doentes apresentaram recorrência de FA (2 com FA paroxística e 9 com FA persistente).
ConclusõesO IVP e parede posterior com PFA mostrou ser um procedimento eficaz e seguro, com baixa taxa de complicações, e reduzida recorrência a curto prazo. Mais estudos são necessários para avaliar o sucesso deste procedimento a longo prazo.
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia in clinical practice, increases in prevalence and incidence with age and is associated with chronic diseases.1,2 It has a well-known relationship with common concomitant cardiovascular diseases, sharing classical cardiovascular risk factors.1,2 AF is a major cause of morbidity and mortality globally, with an increased risk for death, heart failure (HF), hospitalization, and thromboembolic events.3,4
Pulmonary veins (PV) are the primary trigger sites for AF, and PV isolation is currently the gold standard treatment alongside pharmacological treatment. PV isolation should be considered a first line treatment for selected groups of patients as it improves not only quality of life, but also mortality and hospitalizations in patients.5,6
Since its inception, the technique has evolved and several energy sources have been used.7 There are different sources of thermal energy as cryoablation, which showed non-inferiority to radiofrequency.8 Also, the development of irrigated catheters with contact force, as well as the improvement of electroanatomical mapping systems and the development of index-controlled lesions have enabled quicker and safer procedures.9–11 Nevertheless, safety considerations related to all thermal energy-based systems remain, including pericardial tamponade, PV stenosis, atrio-esophageal fistula, and phrenic nerve palsy.12,13
Pulsed field ablation (PFA) is a recent nonthermal energy that destabilizes cell membranes leading to a cellular apoptosis-like process.14,15 It involves the application of ultra-rapid electrical impulses causing irreversible nanoscale pore perforation and consequently, cellular death.14 By optimizing parameters such as voltage amplitude, phasic waveforms and pulse sequences, PFA can selectively target the myocardial tissue, avoiding lesions to peri-cardiac muscular and nervous structures such as the esophagus or phrenic nerve.16 Since it is a novel approach to cardiac ablation of AF with the ability of tissue preference ablation,17 it enables efficient atrial myocardium ablation, while reducing the risk of collateral tissue damage.16,17
ObjectiveIn this original research, we share our one-year experience of paroxysmal and persistent AF ablation, using the multielectrode pentaspline PFA catheter (FARAPULSE Boston Scientific).
MethodsStudy population, clinical data, and study designThis was a single center retrospective study that included patients with AF, over 18 years of age, and electively referred for symptomatic AF ablation, between June 2022 and July 2023.
Patients were excluded from the procedure if they had AF secondary to electrolyte imbalance, thyroid disease, or reversible or non-cardiac cause; left atrial thrombus or a contraindication for anticoagulation.
Clinical parameters – demographics, major cardiovascular risk factors, symptomatic status – were collected.
Pulsed field ablation procedure workflowPrior to the procedure, all patients underwent cardiac computed angiography for atrial and pulmonary vein assessment, and to rule-out left atrial appendage thrombus.18 Treatment with uninterrupted systemic anticoagulation therapy was required for at least three weeks prior to study treatment. During the ablation procedure, unfractionated heparin was administered right before transseptal puncture to achieve and maintain an activated clotting time of between 300 and 350 seconds.19 Ultrasound guidance was used to obtain femoral venous access.
Ablation was performed under fluoroscopy guidance with or without the use of a three-dimensional electroanatomic mapping system (CARTO 3, BiosenseWebster, Diamond Bar, CA, USA). All ablation procedures included were managed with anesthesiology on site. They were performed either under conscious deep sedation using continuous remifentanil and propofol infusion or general anesthesia, at the physician's discretion. In this approach, coughing, patient movement or patient discomfort were not an issue.
During the PFA procedure, a 6F quadripolar catheter was used to pace the right ventricle as needed during periods of asystole.
The novel multielectrode pentaspline PFA catheter (FARAPULSE Boston Scientific) was used. Catheters were placed in the coronary sinus and his bundle region/right ventricle. As outlined in the MANIFEST-PF trial,17 PFA protocol comprises a pentaspline PFA catheter (Farawave, 12-Fr) inserted through a 13-Fr steerable sheath with a transparent shaft (Faradrive) into the left atrium (LA). Once the recommended straight-tip 0.035 guidewire (Amplatz extra stiff straight wire; Cook Inc.) is positioned in each target PV, the PFA catheter is placed at the ostium of each PV to apply a total of eight PF lesions per vein. Additional lesions were performed on larger veins or pulmonary trunk whenever abnormal signals were found. The lesions are distributed in ‘basket’ and ‘flower’ configurations, with rotation between each pair of lesions. For ablation of the posterior LA wall, the catheter is deployed in the flower configuration and placed along the posterior LA to deliver overlapping sets of pulses at each location. The pulse field voltage amplitude can vary between 1.8 and 2.0 kV, with 2.0 kV typically being used. The total procedure time was the duration from obtaining vascular access to the removal of catheters from the patient.
According to the hospital's policy, patients are scheduled for a follow-up appointment usually at three months after AF ablation. Oral anticoagulation therapy was performed at least until this reassessment and adjusted according to the CHA2DS2-VASc score.5 The administration of antiarrhythmic drugs after ablation was at the treating physician's discretion.
Outcomes and follow-upThe primary effectiveness outcome was acute success, defined as first pass isolation (PW and PV isolation).
The secondary effectiveness outcome was relapse of any atrial arrhythmia after a three-month blanking period, and was defined as the presence of AF, atrial flutter, or any atrial arrhythmia on routine electrocardiogram (ECG) or 24-hours Holter test, extracted from electronic medical records.
The main safety endpoint was a composite of procedure-related death, atrioesophageal fistula, cardiac tamponade, myocardial infarction, cerebrovascular events, persistent phrenic nerve injuries, PV stenosis or vascular access complications.
Statistical analysisCategorical values are presented as absolute numbers (and percentage). Continuous variables as mean±standard deviation (normal distribution) or as median and interquartile range (IQR; non-parametric). Kolmogorov–Smirnov test was used to test normality of the variables.
Independent sample T-test and Mann–Whitney tests were applied for comparison where appropriate. Statistical significance was set at p-value <0.05 (two-sided). All analyses were performed using Statistical Package for the Social Sciences Statistics v27.0 (IBM Corporation, Armonk, NY, USA).
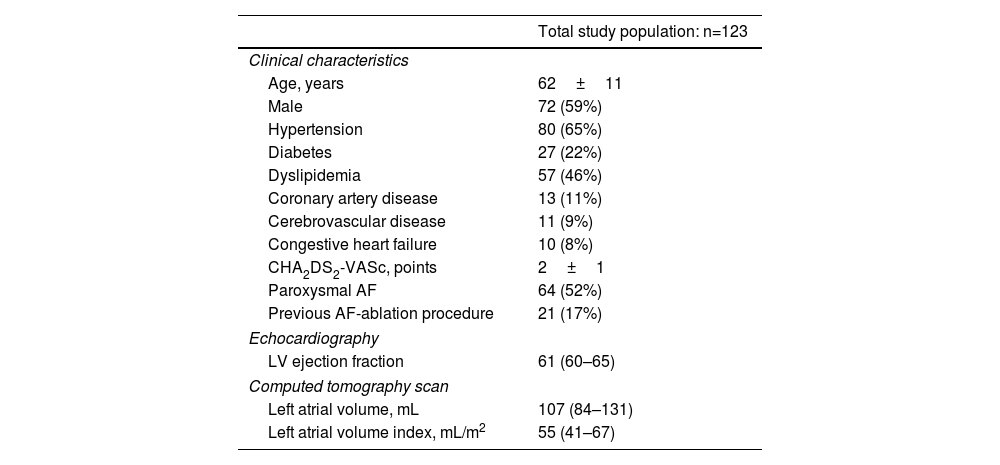
ResultsStudy population and clinical dataOverall, we studied 123 consecutive patients who were electively referred for symptomatic AF ablation, aged 62±11 years, 72 (59%) male, mean CHA2DS2-VASc score of 2±1 points. Fifty-two percent of patients presented paroxysmal AF, and 21 patients (17%) had undergone a previous ablation procedure. No statistical differences were found in comparative analyses among paroxysmal and non-paroxysmal AF redo patients (Supplemental Table 1). At transthoracic echocardiogram evaluation, a median left ventricular ejection fraction of 61% [IQR 60–65%] was found.
Regarding cardiovascular risk factors and comorbidities: 65% (n=80) had a previous diagnosis of hypertension; 22% (n=27) patients had diabetes and 8% (n=10) presented congestive HF. Demographic and clinical data are summarized in Table 1. All patients were under uninterrupted systemic anticoagulation therapy for at least three weeks prior to study treatment.
Baseline demographic and clinical data of the study cohort.
| Total study population: n=123 | |
|---|---|
| Clinical characteristics | |
| Age, years | 62±11 |
| Male | 72 (59%) |
| Hypertension | 80 (65%) |
| Diabetes | 27 (22%) |
| Dyslipidemia | 57 (46%) |
| Coronary artery disease | 13 (11%) |
| Cerebrovascular disease | 11 (9%) |
| Congestive heart failure | 10 (8%) |
| CHA2DS2-VASc, points | 2±1 |
| Paroxysmal AF | 64 (52%) |
| Previous AF-ablation procedure | 21 (17%) |
| Echocardiography | |
| LV ejection fraction | 61 (60–65) |
| Computed tomography scan | |
| Left atrial volume, mL | 107 (84–131) |
| Left atrial volume index, mL/m2 | 55 (41–67) |
Values are median (interquartile range), mean±standard deviation. AF: atrial fibrillation; LV: left ventricle.
As previously stated, all patients performed a CT scan before ablation procedure for PV mapping, and to rule-out LA appendage thrombus, with a median CT scan derived left atrial volume index of 55 mL/m2 (IQR 41–67 mL/m2). All procedures were performed with only one transeptal puncture.
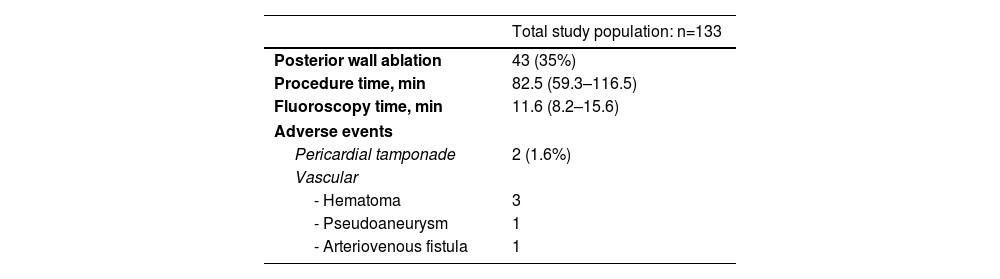
Electroanatomical mapping was performed in 88 patients (n=72%) – all with CARTO®3D – in relation to the initial experience of the operators and, mainly, when posterior wall isolation (PWI) was planned. All patients underwent PVI using the pentaspline PFA catheter. Of the 123 participants who underwent ablation, PVI was performed in all of them. PWI was performed in 43 patients (35%), based on the operator's description, on LA volume and primarily guided by the assessment of fragmented potentials and/or left atrial fibrosis. The median procedure time was 83 min [IQR 59–117 min]. Of this total procedure time, fluoroscopy time was 11.6 min [IQR 8.2–15.6 min] – Table 2. Comparing these times in the first half of the procedures performed with those in the following months, it was found that the procedure [89 (IQR 68–119) vs. 77 (IQR 46–113) min, p=0.023] and fluoroscopy [13 (IQR 10–16) vs. 10 (IQR 8–13) min, p=0.005] times were significantly longer in the initially performed procedures. In a comparative analysis based on different workflow approaches (Supplemental Table 2): fluoroscopy-guided PVI; three dimensional (3D) mapping-guided PVI; fluoroscopy-guided PVI+PWI (if used); and 3D mapping-guided PVI+PWI, we found a significant statistical difference related to the procedure (p<0.001) and fluoroscopy (p=0.022) times. Patients who underwent fluoroscopy-guided PVI presented the lowest median procedure time and the lowest median fluoroscopy time.
Ablation procedure data of the study cohort.
| Total study population: n=133 | |
|---|---|
| Posterior wall ablation | 43 (35%) |
| Procedure time, min | 82.5 (59.3–116.5) |
| Fluoroscopy time, min | 11.6 (8.2–15.6) |
| Adverse events | |
| Pericardial tamponade | 2 (1.6%) |
| Vascular | |
| - Hematoma | 3 |
| - Pseudoaneurysm | 1 |
| - Arteriovenous fistula | 1 |
Values are median (interquartile range), mean±standard deviation.
Among patients who underwent redo-AF ablation, only reconnected PVs were isolated, with a mean of 2±1 reconnected veins per patient.
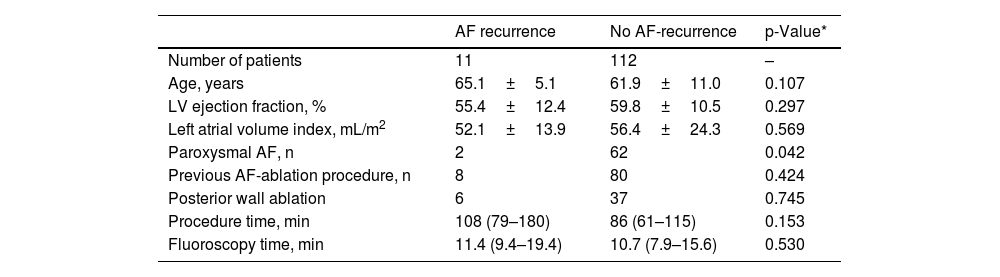
EffectivenessThe primary effectiveness endpoint (defined as first pass isolation – PW and PVI) was achieved in 100% of patients. Over 290 (IQR 169–387) days of follow-up, 112 patients (91%) were in sinus rhythm, whereas 9% of patients had AF recurrence (two with paroxysmal AF and nine with persistent AF). Basal and procedural characteristics were similar between the relapse and the non-relapse group (Table 3). In addition to age, no differences were found between patients with paroxysmal and non-paroxysmal AF with recurrence of AF during follow-up (Supplemental Table 3).
Comparative analyses between patients with atrial fibrillation recurrence on follow-up and no AF recurrence.
| AF recurrence | No AF-recurrence | p-Value* | |
|---|---|---|---|
| Number of patients | 11 | 112 | – |
| Age, years | 65.1±5.1 | 61.9±11.0 | 0.107 |
| LV ejection fraction, % | 55.4±12.4 | 59.8±10.5 | 0.297 |
| Left atrial volume index, mL/m2 | 52.1±13.9 | 56.4±24.3 | 0.569 |
| Paroxysmal AF, n | 2 | 62 | 0.042 |
| Previous AF-ablation procedure, n | 8 | 80 | 0.424 |
| Posterior wall ablation | 6 | 37 | 0.745 |
| Procedure time, min | 108 (79–180) | 86 (61–115) | 0.153 |
| Fluoroscopy time, min | 11.4 (9.4–19.4) | 10.7 (7.9–15.6) | 0.530 |
Values are median (interquartile range), mean±standard deviation. AF: atrial fibrillation; LV: left ventricle.
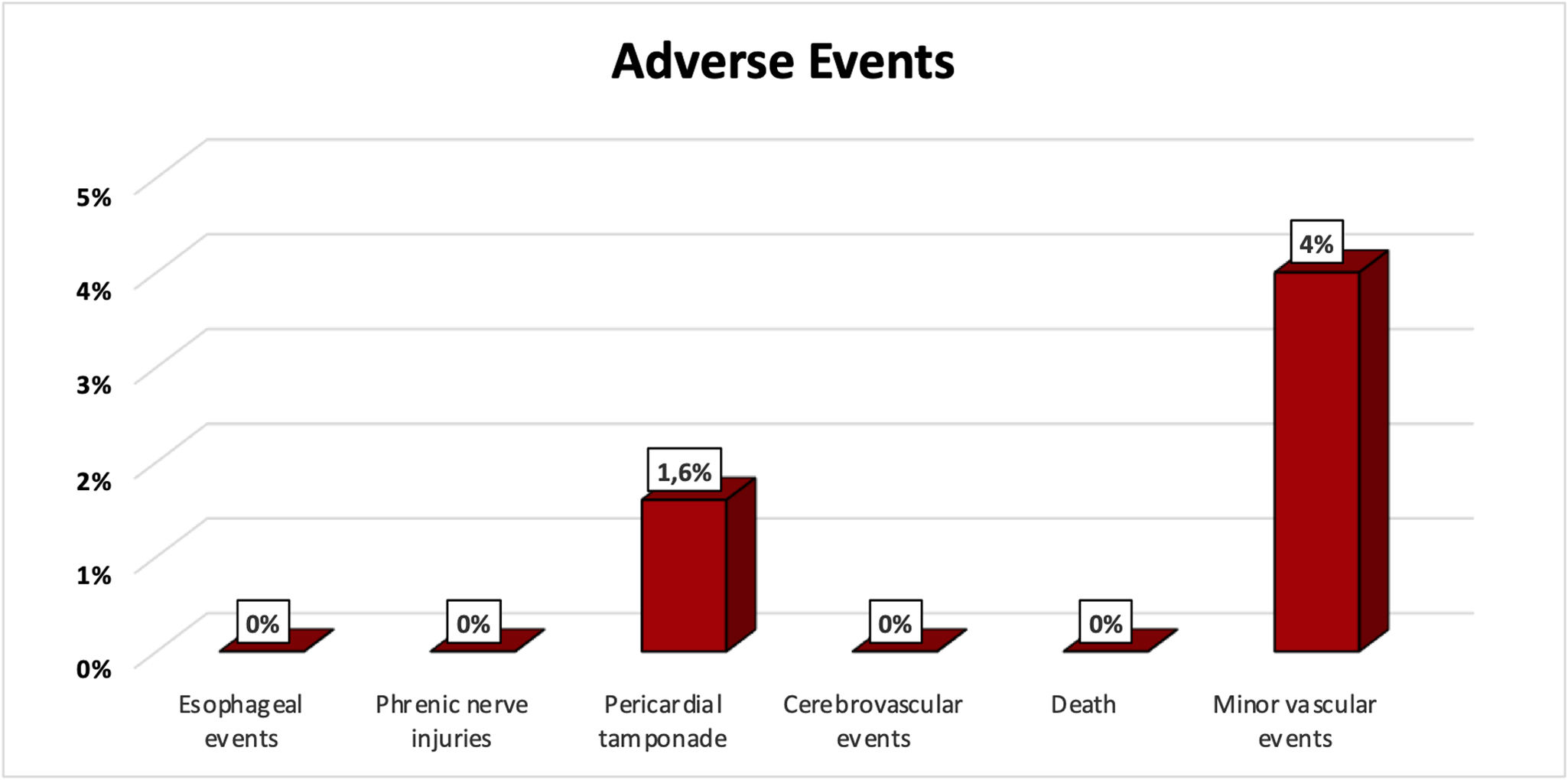
There were no esophageal complications, persistent phrenic nerve injuries, cerebrovascular events, myocardial infarction, PV stenosis or procedure-related deaths (Figure 1). There was no evidence of ST-segment elevation, neither coronary spasm, arrhythmias, or ventricular wall motion abnormalities.
Overall, the number of PFA applications was less than 80, with no cases of hemolysis being reported.
Two patients (1.6%) experienced acute cardiac tamponade. While the causes were not easy to identify, both these procedures were done early in the center experience, and in both cases, the Farawave™ catheter was removed from Faradrive™ sheath and the LA was remapped with a Pentaray® catheter after ablation. As such, they were attributed to catheter manipulation. Both complications were managed with pericardiocentesis, without the need for cardiac surgery. The other complications were vascular, in 4% of cases: three femoral hematomas, one femoral pseudoaneurysms and one arteriovenous fistula – all treated with conservative measures. Also, the hematoma complications were minor as they did not prolong hospitalization.
DiscussionThis study describes our initial experience of a novel ablation modality that involves the application of electrical pulses causing induced cellular death, with preferential tissue specificity (PFA procedure) in a cohort of patients electively referred for symptomatic AF ablation. Our main findings were: (1) PFA for PVI and posterior wall ablation is effective; (2) PFA technique had a low rate of intraprocedural and peri/post-procedural complications; (3) in our cohort, PFA had a high percentage of patients who remained free from AF in the short-term.
Evidence supporting PFA procedure is still scarce and only limited real-world data have been reported, mainly concerning procedure complications and longitudinal data following intervention. We believe our study adds to the current body of knowledge, describing our initial experience with the novel technique, addressing the procedure, the complications, and follow-up following intervention.
Compared to prior PFA studies,17,20 although a relatively short procedure duration was observed in our cohort, it was slightly longer than previous reports. Nevertheless, both procedure and fluoroscopy times in the first half of the procedures performed were significantly longer than those performed in the following months. This highlights the phenomenon of the learning curve, suggesting that more experienced operators can perform the procedure more efficiently and with less fluoroscopy.
Anatomical mapping was conducted in a substantial number of patients. During the first ablation procedures, which were PVI alone, we used anatomical mapping to confirm the success of PVI. However, we no longer perform it systematically, as PVI was successful in all initial cases. For redo procedures, we believe that mapping systems can enhance the accuracy of identifying gaps and achieving better PVI outcomes. In patients with persistent AF and low voltage areas who are candidates for PWI, we find that mapping systems are valuable tools for achieving complete PWI, a challenge with earlier RFA techniques.
Effective PV isolation is one of the major determining factors for the efficacy of PFA and, consistent with some previously reported PFA results,17,21 we achieved 100% intraprocedural technical success. Until recently, PVI has mainly been performed using traditional thermal techniques such as radiofrequency, cryotherapy, or laser therapy. However, research has shown that these methods can cause damage to surround tissues, including the esophagus, phrenic nerve, and aorta.21–23 These thermal techniques induce coagulative necrosis and subsequent reparative fibrosis, which can lead to pulmonary vein narrowing and reduced left atrial function.24 In contrast, PFA is a novel approach to treating AF through a nonthermal method that involves using short electrical pulses to create pores in cell membranes, resulting in selectively myocardial cell death.22,25 This is a potential reason why there was no requirement for an esophageal ‘management strategy’ during the PFA procedures. According to this technical procedure assumption, in our work we found a low rate of intraprocedural and peri/post-procedural complications, like the complication rates reported in RF and cryo-procedures.
In addition to safety, and despite the short follow-up time, we also observed that PFA is an effective ablation technique with a low percentage of long-term recurrence. The fact that the majority of the procedures were performed with electroanatomical mapping may have influenced our results. However, these findings need further investigation.
Our study had some limitations. It was conducted at a single tertiary center with a moderate sample size, thus limiting the extrapolation of the results. Additionally, the post-ablation therapy antiarrhythmic drugs schemes, as well as the number of ECGs/Holter tests were not standardized and were left to each assistant cardiology discretion, which may have influenced our results.
Finally, additional studies with longer-term follow-up and more robust arrhythmia monitoring are required to verify the long-term effectiveness and correlations between short-term follow-up and arrhythmia recurrence.
ConclusionPulsed field ablation for PVI and posterior wall ablation was an efficient and safe procedure, had a low rate of complications and high percentage of patients free from AF in the first year of follow-up.
Ethical approvalStudy approval was granted by the ethical committee of Centro Hospitalar de Lisboa Ocidental (number 2117) conforming to the principles of the Helsinki declaration.
FundingThe authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflicts of interestNo conflicts of interest to declare.