Catheter ablation (CA) is effective in the treatment of ventricular tachycardia (VT). Although some observational data suggest patients with non-ischemic cardiomyopathy (NICM) have less favorable outcomes when compared to those with an ischemic etiology (ICM), direct comparisons are rarely reported. We aimed to compare the outcomes of VT ablation in a propensity-score matched population of ICM or NICM patients.
MethodsSingle-center retrospective study of consecutive patients undergoing VT ablation from 2012 to 2023. A propensity score (PS) was used to match ICM and NICM patients in a 1:1 fashion according to age, sex, left ventricular ejection fraction (LVEF), NYHA class, electrical storm (ES) at presentation, and previous endocardial ablation. The outcomes of interest were VT-free survival and all-cause mortality.
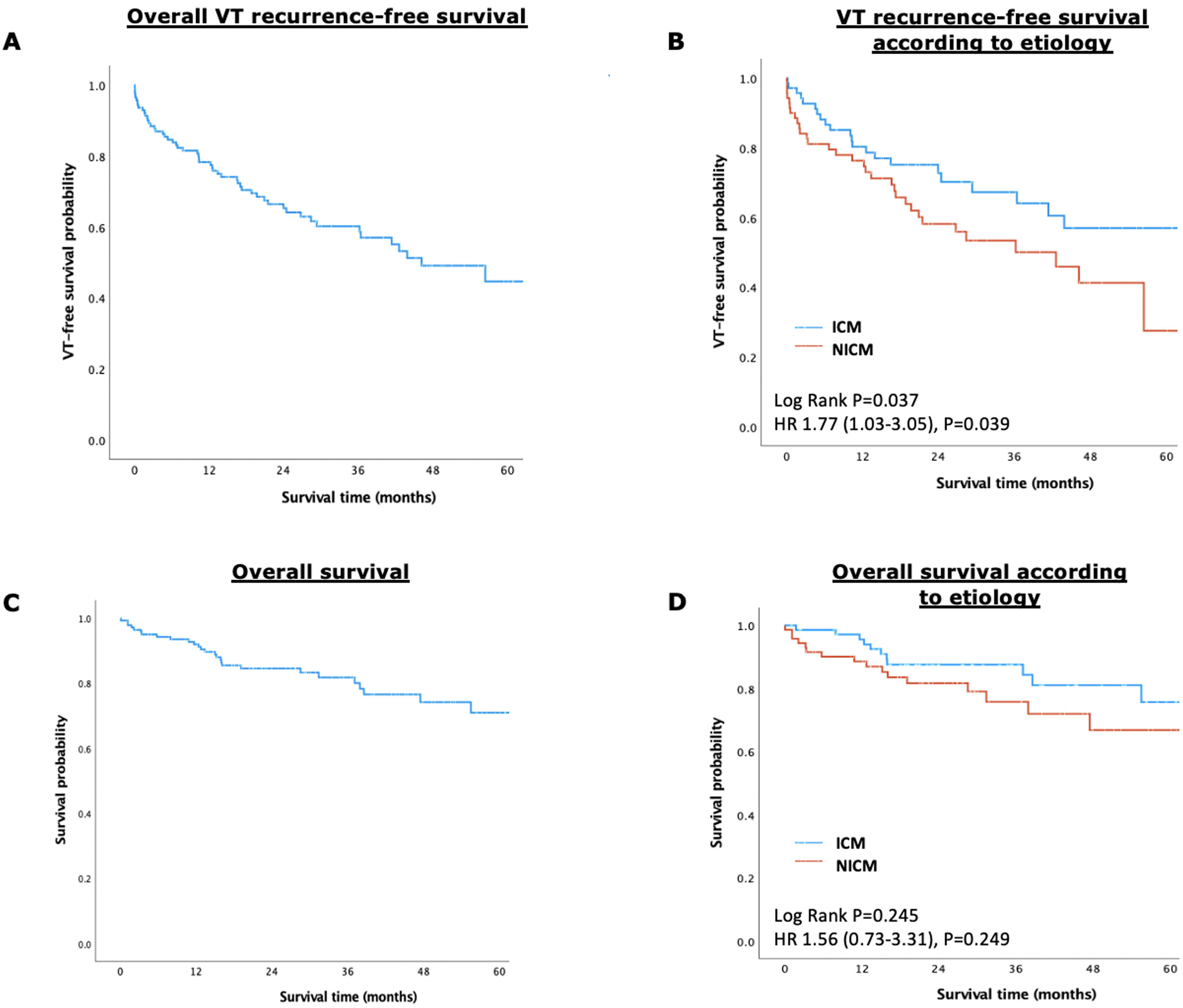
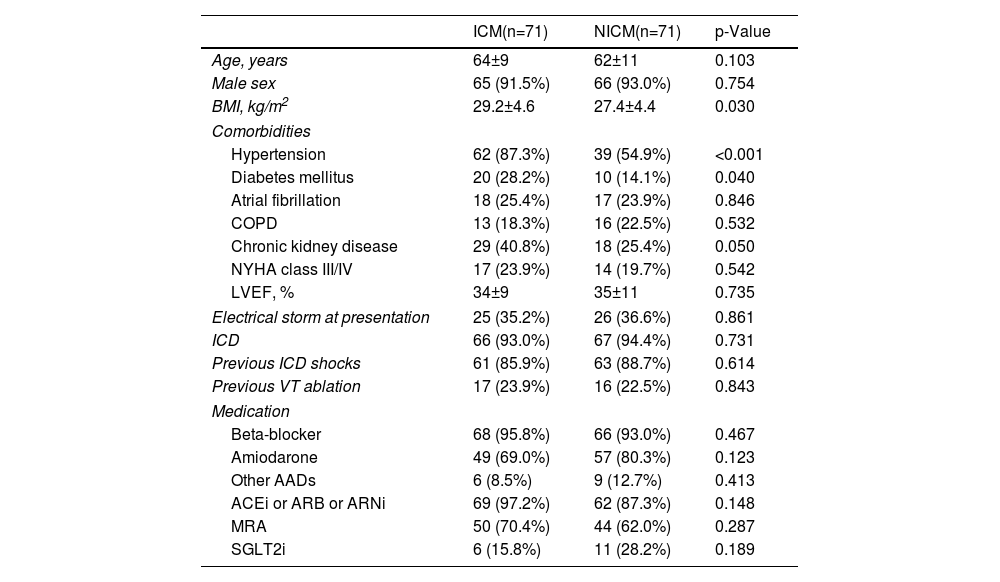
ResultsThe PS yielded two groups of 71 patients each (mean age 63±10 years, 92% male, mean LVEF 35±10%, 36% with ES at presentation, and 23% with previous ablation), well matched for baseline characteristics. During a median follow-up of 2.3 (interquartile range IQR 1.3–3.8) years, patients with NICM had a significantly lower VT-free survival (53.5% vs. 69.0%, log-rank p=0.037), although there were no differences regarding all-cause mortality (22.5% vs. 16.9%, log-rank p=0.245). Multivariate analysis identified NICM (HR 2.34 [95% CI 1.32–4.14], p=0.004), NYHA class III/IV (HR 2.11 [95% CI 1.11–4.04], p=0.024), and chronic kidney disease (HR 2.23 [95% CI 1.25–3.96], p=0.006), as independent predictors of VT recurrence.
ConclusionNon-ischemic cardiomyopathy patients were at increased risk of VT recurrence after ablation, although long-term mortality did not differ.
A ablação por cateter (CA) é eficaz no tratamento da taquicardia ventricular (TV). Embora dados observacionais sugiram que doentes com miocardiopatia não isquémica (NICM) apresentem piores resultados do que aqueles com etiologia isquémica (ICM), comparações diretas são escassamente reportadas. O objetivo foi comparar os resultados da ablação de TV numa população propensity-matched de doentes com NICM ou ICM.
MétodosEstudo retrospetivo unicêntrico de doentes submetidos ablação de TV, de 2012 a 2023. Usado propensity-score (PS) para emparelhar doentes com NICM e ICM numa proporção 1:1 de acordo com idade, sexo, fração de ejeção ventricular esquerda (FEVE), classe de NYHA, tempestade arrítmica à admissão e ablação endocárdica prévia. Os outcomes de interesse foram sobrevida livre de TV e morte por todas as causas.
ResultadosO PS resultou em dois grupos de 71 doentes (idade 63 ± 10 anos, 93% do sexo masculino, FEVE 35 ± 10%, 36% com apresentação em tempestade arrítmica e 23% com ablação prévia). Durante um follow-up de 2,3 (1,3–3,8) anos, os doentes com NICM apresentaram menor sobrevida livre de TV (53,5% versus 69,0%, P log-rank = 0,037), apesar de não haver diferenças significativas em relação à mortalidade (22,5% versus 16,9%, P log-rank = 0,245). A análise multivariada identificou NICM (HR 2,34 [IC 95% 1,32–4,14], P = 0,004), NYHA III ou IV (HR 2,11 [IC 95% 1,11–4,04], P = 0,024) e doença renal crónica (HR 2,23 [IC 95% 1,25–3,96], P = 0,006) como preditores independentes de recidiva de TV.
ConclusãoDoentes com NICM apresentam maior risco de recidiva de TV, apesar de não haver diferenças significativas na mortalidade a longo prazo.
Ventricular tachycardia (VT) poses a significant threat to patients with both ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM), contributing substantially to morbidity and mortality in these populations.1 While implantable cardioverter defibrillators (ICDs) are a crucial tool to prevent sudden death from VT, they have no role in preventing recurrent VT episodes.2 Moreover, the administration of ICD shocks, whether appropriate or inappropriate, can adversely affect both quality of life and long-term survival.3–5 On the other hand, medical therapy with anti-arrhythmic drugs offers limited efficacy in preventing VT recurrence and may carry the risk of severe side effects.6
In response to these challenges, catheter ablation (CA) has emerged as a pivotal intervention to control and prevent recurrent sustained VT episodes.7–10 Due to scientific and technological advancements in recent decades, including procedural planning utilizing advanced imaging techniques and its integration with 3D mapping systems, the widespread use of high-density mapping catheters, and new algorithms for the automatic annotation of electrograms, VT ablation is currently a safe and effective percutaneous catheter-based therapy.11,12 It targets myocardial tissue from either the endocardial and/or epicardial surfaces, aiming to identify critical VT isthmuses.13,14
In the setting of structural heart disease (SHD), current guidelines recommend VT ablation for patients who have failed or cannot tolerate anti-arrhythmic therapy.15–17 These recommendations derive from multiple observational reports and randomized trials in ICM patients with VT.7,18,19 Only a few small sample studies have addressed the outcomes in NICM patients, mostly reporting worse outcomes.20,21 Poor outcomes may partly be attributed to their distinct substrate characteristics regarding the location of scar tissue, as well as the progressive nature of the disease.22,23
Uncertainties persist regarding the predictors of both short- and long-term success following VT ablation, with conflicting results emerging from studies focused on ICM populations.18,19,24,25 Notably, while some trials have identified acute VT non-inducibility as an independent predictor of long-term recurrence-free survival, others have failed to replicate these findings.26,27 Data regarding NICM are even scarcer, and outcomes vary greatly according to the inclusion criteria.21,28 Furthermore, direct comparisons between NICM and ICM populations have yielded inconclusive results, suggesting similar short-term outcomes but potentially worse long-term prognoses for NICM patients.2,23,29 Hence, robust evidence to support these findings is necessary.
ObjectiveThe aim of our study was to compare the outcomes of VT ablation in a propensity-score matched population of ICM or NICM patients.
MethodsStudy populationThis was a single-center retrospective study including consecutive patients with SHD undergoing VT ablation from 2012 to 2023. Only those with scar-related VT of either ICM or dilated cardiomyopathy/non-dilated left ventricular cardiomyopathy etiology were considered. Alternative cardiomyopathies, including Chagas disease, sarcoidosis, right ventricular arrhythmogenic cardiomyopathy, hypertrophic cardiomyopathy, and acute myocarditis were excluded. In all cases, VT was documented using cardiac implantable electronic devices, 12-lead electrocardiogram (ECG), or external defibrillator monitoring. The definitions of ischemic and non-ischemic cardiomyopathy were according to current international guidelines.15,17 Electrical storm was defined as the occurrence of ≥3 episodes of sustained VT or ventricular fibrillation during a 24-hour period. This study was according to the amended Declaration of Helsinki. All patients signed an informed consent, and the registry had been previously approved.30
Electrophysiology procedureAll patients underwent the procedure under conscious sedation or general anesthesia according to the operator and anesthesiologist's judgment. Whenever clinically appropriate, anti-arrhythmic drugs (AADs) were withheld at least 48 hours before the VT ablation. Anticoagulants were stopped 24–48 hours prior depending on renal function and/or INR level (if under vitamin K antagonist) and resumed within the first 24 hours in the absence of any hemorrhagic complications. The choice of the ablation access (endocardial, epicardial or combined) was left to the operator's discretion and decided according to etiology, previous ablation site, and imaging information regarding scar location.
A decapolar steerable catheter was placed in the coronary sinus via the femoral vein and a quadripolar non-steerable catheter was placed in the right ventricular apex. Left ventricular (LV) endocardial mapping was performed either via retrograde aortic or transseptal route according to the operator's discretion. After LV access was obtained, systemic anticoagulation with unfractionated heparin was administered, targeting a minimum activation clotting time of 300 s during endocardial ablation. Anticoagulation was reversed with protamine sulfate (1 mg/100 U) at the end of the procedure or before percutaneous epicardial access, when needed. If clinical or preprocedural imaging features suggested an epicardial circuit, access was obtained through the subxiphoid space under fluoroscopic guidance as previously described by Sosa et al.31
Substrate and tachycardia mappingElectroanatomical maps were obtained with the CARTO® (Biosense Webster, Diamond Bar, CA, USA), EnSite NavX® (Abbot, St Paul, MN, USA), or Rhythmia® (Boston Scientific, Marlborough, Massachusetts, USA) systems and included activation and voltage mapping acquired in sinus rhythm, ventricular pacing or during hemodynamically stable VT. Mapping catheters used included a 20-electrode PentaRay® catheter (Biosense Webster), a 16-electrode HDGrid® catheter (Abbot), or a 64-electrode Intellamap Orion® basket catheter (Boston Scientific), respectively. Catheters were supported by an Agilis® steerable sheath (Abbot) during anterograde mapping.
An attempt to induce the clinical VT was performed at the beginning of each procedure according to the clinical characteristics of the patients and the operator's discretion. Clinical VT was defined as an inducible VT with a similar cycle length (CL) of stored ICD electrograms and/or the 12-lead ECG morphology and CL when available. When hemodynamically tolerated, activation and entrainment mapping were used to define critical components of the circuit. Substrate mapping was performed during normal sinus rhythm or pacing. Normal tissue was defined by a bipolar voltage threshold greater than 1.5 mV, while dense scar as <0.5 mV (in extensive areas of low voltage, a cut-off of 0.3 mV – or as low as 0.1 mV – was used to better discriminate slow conduction zones). Local abnormal ventricular activities (LAVA) were defined as sharp high-frequency ventricular potentials, distinct from the far-field ventricular electrogram, occurring during or after the ventricular electrogram.32
Catheter ablationRadiofrequency (RF) was delivered with a 4 mm irrigated and contact force monitoring ablation catheter in power control mode, with power set to 30–50 W and irrigation set to 17–30 ml/min. In one patient with ICM, pulsed-field ablation (FARAPULSE®: FARAWAVE catheter and FARADRIVE steerable sheath; Boston Scientific) was also utilized. In the presence of sustained and tolerated monomorphic VT, isthmus was the first target for ablation. If clinical VT was not inducible or mappable, substrate modification was performed in sites in which abnormal electrograms were identified.
The procedure was considered acutely successful if no VT was induced with a standard stimulation protocol of up to three extrastimuli at a cycle length of down to 200 ms or ventricular effective refractory period from two different sites, excluding induction of VF or polymorphic VT.30 Inducibility testing was withheld if patients were hemodynamically unstable, or if repeated cardioversions were required for hemodynamically unstable VT during the procedure.
Study endpointsThe primary outcome of interest was VT-free survival. VT recurrence included any ICD-appropriate therapy (anti-tachycardia pacing or shock), or a documented sustained VT not detected by the ICD. The secondary endpoint was all-cause mortality.
Follow-upThe follow-up comprised regular outpatient visits according to the assistant physician's discretion. Outcomes were collected from patients’ electronic medical records, ICD home monitoring software, and a structured telephonic interview whenever necessary. All patients without an ICD underwent implantation before discharge. After the ablation, all ICD were programmed with a detection zone slower than the clinical VT. Vital status was determined for each patient using the Portuguese National Registry.
Statistical analysisCategorical variables were reported as numbers and percentages. Continuous variables were described as means and standard deviations for normally distributed variables and medians and interquartile ranges for non-normally distributed variables. Clinical characteristics of the subgroups of interest were compared using the χ2-test and Fisher's exact test (when applicable) for dichotomous variables; and the unpaired Student's t-test or Mann–Whitney U test (when applicable) for continuous variables. A propensity score (PS) was applied to match each patient of the ICM cohort with a similar subject in the NICM group, in a 1:1 fashion. The following baseline variables, known to impact the results and safety of the procedure, were included the propensity score model: age, sex, left ventricular ejection fraction (LVEF), New York Heart Association (NYHA) class, electrical storm (ES) at presentation, and previous endocardial VT ablation.33–36 Patients were matched on the logit of the propensity score using calipers of width equal to 0.1 of the standard deviation. Anti-arrhythmic drugs were not included in the main analysis as time points for their collection were different in each group. Kaplan–Meier survival curves were plotted to compare groups, and the log-rank test was used to assess for significant differences in time to endpoint between groups. Cox proportional hazards regression was applied for univariable and multivariable analysis to investigate the association between clinical variables with VT recurrence. Variables with a p<0.05 or theoretically relevant (domain knowledge) were included in the multivariable model. A multivariate Cox regression model for the outcome of VT recurrence in the entire non-matched population was performed as a sensitivity analysis. A two-tailed p<0.05 was considered statistically significant for all tests. All analyses were performed using SPSS v26.0 (IBM Corporation, Armonk, New York) and STATA v13.0 (StataCorp, College Station, Texas).
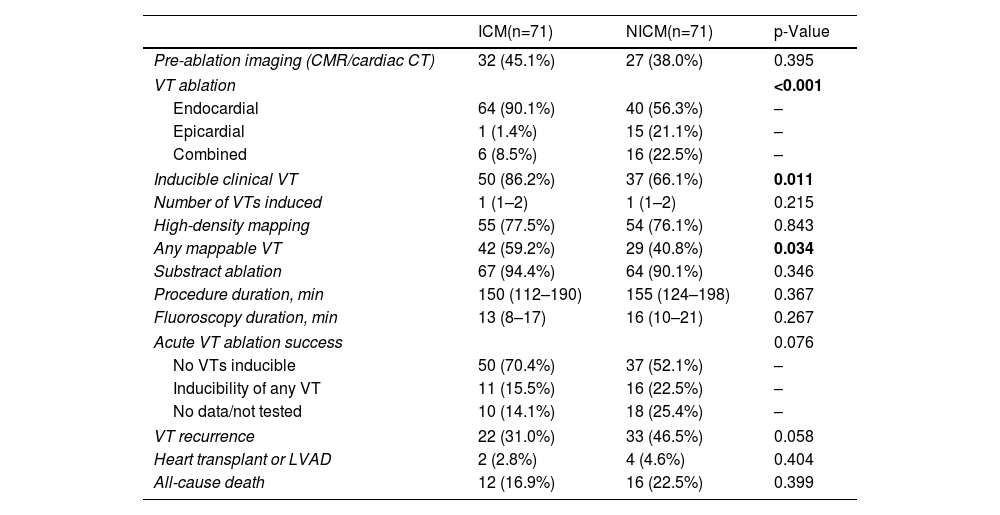
ResultsBaseline characteristicsOf the included 248 patients, the PS model yielded two groups of 71 patients each, well matched for baseline variables. Briefly, the mean age of the included population was 63±10 years, 92% (n=131) were male, mean left ventricular ejection fraction (LVEF) was 35±10% and 36% (n=51) of patients had electrical storm (ES) at presentation. A total of 23% (n=33) had history of previous endocardial ablation. The baseline characteristics are presented in Table 1.
Clinical baseline characteristics of the propensity-matched cohort.
| ICM(n=71) | NICM(n=71) | p-Value | |
|---|---|---|---|
| Age, years | 64±9 | 62±11 | 0.103 |
| Male sex | 65 (91.5%) | 66 (93.0%) | 0.754 |
| BMI, kg/m2 | 29.2±4.6 | 27.4±4.4 | 0.030 |
| Comorbidities | |||
| Hypertension | 62 (87.3%) | 39 (54.9%) | <0.001 |
| Diabetes mellitus | 20 (28.2%) | 10 (14.1%) | 0.040 |
| Atrial fibrillation | 18 (25.4%) | 17 (23.9%) | 0.846 |
| COPD | 13 (18.3%) | 16 (22.5%) | 0.532 |
| Chronic kidney disease | 29 (40.8%) | 18 (25.4%) | 0.050 |
| NYHA class III/IV | 17 (23.9%) | 14 (19.7%) | 0.542 |
| LVEF, % | 34±9 | 35±11 | 0.735 |
| Electrical storm at presentation | 25 (35.2%) | 26 (36.6%) | 0.861 |
| ICD | 66 (93.0%) | 67 (94.4%) | 0.731 |
| Previous ICD shocks | 61 (85.9%) | 63 (88.7%) | 0.614 |
| Previous VT ablation | 17 (23.9%) | 16 (22.5%) | 0.843 |
| Medication | |||
| Beta-blocker | 68 (95.8%) | 66 (93.0%) | 0.467 |
| Amiodarone | 49 (69.0%) | 57 (80.3%) | 0.123 |
| Other AADs | 6 (8.5%) | 9 (12.7%) | 0.413 |
| ACEi or ARB or ARNi | 69 (97.2%) | 62 (87.3%) | 0.148 |
| MRA | 50 (70.4%) | 44 (62.0%) | 0.287 |
| SGLT2i | 6 (15.8%) | 11 (28.2%) | 0.189 |
AAD: anti-arrhythmic drug; ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNi: angiotensin receptor/neprilysin inhibitor; BMI: body-mass index; COPD: chronic pulmonary obstructive disease; ICD: implantable cardioverter defibrillator; LVAD: left ventricular assist device; LVEF: left ventricular ejection fraction; SGLT2i: sodium-glucose cotransporter-2 inhibitor; VT: ventricular tachycardia. Chronic kidney disease defined as CKD-EPI eGFR <60 ml/min/1.73 m2.
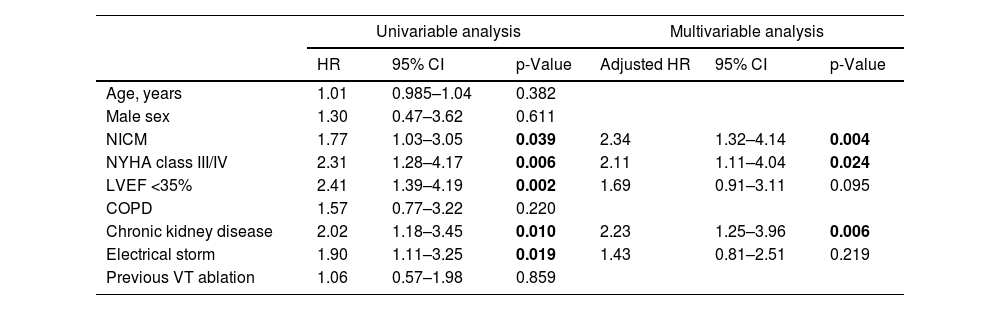
The characteristics related to the ablation procedure are depicted in Table 2. Patients with NICM were more likely to undergo either epicardial or combined VT ablation (43.7% vs. 9.9%, p<0.001). In the 114 patients in which programmed ventricular stimulation was performed at the end of the procedure, non-inducibility of any VT was achieved in 76% (n=87). Non-inducibility was numerically higher in the ICM cohort (50/71 patients vs. 37/71), despite not meeting statistical significance (p=0.076).
VT ablation-related characteristics.
| ICM(n=71) | NICM(n=71) | p-Value | |
|---|---|---|---|
| Pre-ablation imaging (CMR/cardiac CT) | 32 (45.1%) | 27 (38.0%) | 0.395 |
| VT ablation | <0.001 | ||
| Endocardial | 64 (90.1%) | 40 (56.3%) | – |
| Epicardial | 1 (1.4%) | 15 (21.1%) | – |
| Combined | 6 (8.5%) | 16 (22.5%) | – |
| Inducible clinical VT | 50 (86.2%) | 37 (66.1%) | 0.011 |
| Number of VTs induced | 1 (1–2) | 1 (1–2) | 0.215 |
| High-density mapping | 55 (77.5%) | 54 (76.1%) | 0.843 |
| Any mappable VT | 42 (59.2%) | 29 (40.8%) | 0.034 |
| Substract ablation | 67 (94.4%) | 64 (90.1%) | 0.346 |
| Procedure duration, min | 150 (112–190) | 155 (124–198) | 0.367 |
| Fluoroscopy duration, min | 13 (8–17) | 16 (10–21) | 0.267 |
| Acute VT ablation success | 0.076 | ||
| No VTs inducible | 50 (70.4%) | 37 (52.1%) | – |
| Inducibility of any VT | 11 (15.5%) | 16 (22.5%) | – |
| No data/not tested | 10 (14.1%) | 18 (25.4%) | – |
| VT recurrence | 22 (31.0%) | 33 (46.5%) | 0.058 |
| Heart transplant or LVAD | 2 (2.8%) | 4 (4.6%) | 0.404 |
| All-cause death | 12 (16.9%) | 16 (22.5%) | 0.399 |
CMR: cardiac magnetic resonance; CT: computed tomography. Other abbreviations as in Table 1.
Statistically significant p-values are in bold.
A total of 92 patients (65%) were hospitalized at the time of the ablation, whereas the remaining had their procedures scheduled in an ambulatory setting. The median duration of hospital stay was two (interquartile range (IQR) of 2–7) days. Overall, the procedural complication rate was low (7.0%, n=10) and similar between groups (p>0.50) – Supplementary Table 1.
Ventricular tachycardia-free survival and mortalityDuring a median follow-up of 2.3 (IQR 1.3–3.8) years, 39% (n=55) of patients had VT recurrence, 4% (n=6) underwent heart transplantation or long-term left ventricular assist device (LVAD), and 20% (n=28) died. A total of two patients died during the same hospital admission, none related to procedure-related complications.
Patients with NICM had a significantly higher rate of VT recurrence (26.9%/year vs. 14.2%/year, log-rank p=0.037; HR 1.77 [95% CI 1.03–3.05], p=0.039), although there were no statistically significant differences regarding the outcome of all-cause mortality between groups (9.6%/year vs. 6.2%/year, log-rank p=0.245; HR 1.56 [95% CI 0.76–3.31], p=0.249) – Figure 1.
In the multivariate analysis, including baseline clinical characteristics, NICM remained an independent predictor of time-to-VT recurrence (adjusted HR 2.34 [95% CI 1.32–4.14], p=0.004), even after adjusting for the presence of ES at presentation, heart failure (HF) functional class, LVEF and chronic kidney disease (CKD). NYHA class III or IV (adjusted HR 2.11 [95% CI 1.11–4.04], p=0.024), and CKD (adjusted HR 2.23 [95% CI 1.25–3.96], p=0.006) were the other independent predictors of VT recurrence (Table 3).
Uni and multivariate analysis for the endpoint of time-to-VT recurrence.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | Adjusted HR | 95% CI | p-Value | |
| Age, years | 1.01 | 0.985–1.04 | 0.382 | |||
| Male sex | 1.30 | 0.47–3.62 | 0.611 | |||
| NICM | 1.77 | 1.03–3.05 | 0.039 | 2.34 | 1.32–4.14 | 0.004 |
| NYHA class III/IV | 2.31 | 1.28–4.17 | 0.006 | 2.11 | 1.11–4.04 | 0.024 |
| LVEF <35% | 2.41 | 1.39–4.19 | 0.002 | 1.69 | 0.91–3.11 | 0.095 |
| COPD | 1.57 | 0.77–3.22 | 0.220 | |||
| Chronic kidney disease | 2.02 | 1.18–3.45 | 0.010 | 2.23 | 1.25–3.96 | 0.006 |
| Electrical storm | 1.90 | 1.11–3.25 | 0.019 | 1.43 | 0.81–2.51 | 0.219 |
| Previous VT ablation | 1.06 | 0.57–1.98 | 0.859 | |||
Abbreviations as in Table 1.
Statistically significant p-values are in bold.
Regarding the procedural characteristics, non-inducibility of any VT immediately after ablation (i.e., acute ablation success) was associated with significantly lower rates of VT recurrence during follow-up (15.8%/year vs. 33.0%/year, log-rank p=0.03 – Supplementary Figure 1), despite no differences in all-cause mortality (8.2%/year vs. 7.6%/year, log-rank p=0.949).
Sensitivity analysisThe baseline characteristics of the entire non-matched cohort before PS are depicted in Supplementary Table 2. The predictive value of NICM etiology for time-to-VT recurrence was similar in a sensitivity multivariate model including the entire cohort before matching: aHR 1.87 (95% CI 1.19–2.93), p=0.006 (Supplementary Table 3). In this model, ES (HR 1.77 [95% CI 1.15–2.70], p=0.009), reduced LVEF <35% (HR 1.86 [95% CI 1.17–2.95], p=0.009) and CKD (HR 1.64 [95% CI 1.06–2.54], p=0.027) were the other independent predictors of recurrence.
DiscussionIn this study, we assessed the outcomes of VT ablation in a PS matched population of NICM and ICM. The main results of our analysis can be summarized as follows:
- (i)
Patients undergoing VT ablation remain at high risk of recurrence and death (20%/year and 8%/year, respectively).
- (ii)
NICM patients have higher rates of VT recurrence after ablation, although long-term mortality does not differ significantly from ICM.
The VT recurrence rate and risk of mortality remain notably high even after ablation for both ICM and NICM patients. The occurrence of VT in the spectrum of SHD is intrinsically linked to complex and heterogeneous underlying substrates. Typically, in these scenarios, the responsible reentrant circuits tend to exhibit a large size, multiplicity, and variable scar burden and depth, rendering VT ablation challenging.27,37 A previous meta-analysis that combined data from six randomized trials encompassing 791 patients with ICM showed that VT ablation reduced the incidence of appropriate ICD shocks (RR 0.66; 95% CI 0.47–0.92, p=0.02), despite the absence of benefit for all-cause mortality.38 More recently, the SURVIVE-VT trial enrolled 144 individuals with ICM, randomly assigning them in a 1:1 ratio to either complete endocardial substrate-based CA or anti-arrhythmic drugs (AAD).9 Over 24 months, the primary outcome occurred in 28.2% of patients in the ablation group compared to 46.6% in the AAD group (HR: 0.52; 95% CI: 0.30–0.90; p=0.021), specifically due to a significant reduction in severe treatment-related complications (9.9% vs. 28.8%, HR: 0.30; 95% CI: 0.13–0.71; p=0.006), highlighting the safety of the ablation procedure.9 Moreover, the PARTITA study, which followed 517 patients over 24 months, demonstrated that VT ablation following the first appropriate ICD shock significantly reduced the combined risk of death or worsening HF hospitalization (HR: 0.11; 95% CI, 0.01–0.85; p=0.034).39 Within the setting NICM, data from the International Ventricular Tachycardia Ablation Center Collaborative study involving 780 patients revealed that during a median follow-up of 13 months after ablation, VT recurred in 318 (41%) patients and 137 (18%) died, underscoring the severity of such patients.37 However, the PAUSE-SCD trial enrolling 180 NICM patients demonstrated that early ablation therapy significantly reduced the composite primary outcome of VT recurrence, cardiovascular hospitalization, or death compared to conventional medical therapy (49.3% vs. 65.5% in the control group, HR: 0.58; 95% CI 0.35–0.96; p=0.04). The reduction in adverse outcomes was primarily driven by decreased VT recurrence.40
As mentioned above, direct comparisons between ICM and NICM post-VT ablation outcomes, are scarce. Nevertheless, earlier studies have consistently indicated an elevated risk of VT recurrence in NICM patients during follow-up, which is in accordance with our findings. In the observational HELP-VT study, Dinov et al. demonstrated that, during a median follow-up of 20 months, the cumulative VT-free survival for patients with ICM stood at 43%, significantly higher than the 23% observed in NICM patients (HR 1.73; 95% CI 1.03–2.91; p=0.039).23 Notably, there was also no difference in all-cause mortality between ICM and NICM patients (7.9% and 12.7%, respectively, p=0.307).23 Similarly, in our study, we did not find significant variations in overall mortality among groups, likely related to the existence of other competing risks of death (e.g., LV systolic dysfunction, and other cardiovascular and non-cardiovascular comorbidities).23,41
The differences in the VT recurrence rate between ICM and NICM patients may be driven by a variety of factors. VT in these populations presents with distinct anatomic substrates and electrophysiological characteristics. In ICM, scar tissue is often accessible from the endocardium, facilitating endocardial ablation procedures. Conversely, in NICM, scar tissue areas containing reentry circuits are frequently patchy and often located along the base of the LV, in the mid-myocardium or sub-epicardium.42,43 In our study, this is reflected by a four-fold increase in the use of epicardial or combined approaches in the NICM cohort. Furthermore, cardiomyopathies are heterogeneous entities with a progressive nature, and late recurrence can be expected.23 Although VT ablation can modify the existing substrate at the time of the procedure, it will not impede NICM progression and the formation of new substrates. The concept of a “fixed” underlying substrate is possibly true for the ICM only.23
Besides NICM, we have also found chronic kidney disease and NYHA functional class III or IV as independent predictors of recurrence. Despite this, previous reports have shown that ablation can be safely performed in such population, and that early VT relapse is associated with subsequent death regardless of NYHA status.44 Furthermore, elimination of VT recurrence in NYHA IV patients may reduce its mortality to a level comparable to NYHA II and III with arrhythmia recurrence.44 On the other hand, non-inducibility of any VT after ablation was associated with a two-fold recurrence reduction during follow-up. Interestingly, while some trials involving ICM patients have identified acute VT non-inducibility as an independent predictor of long-term VT-free survival, others have not replicated these findings consistently.18,27,45 Within the NICM population, several studies have struggled to establish non-inducibility as a reliable predictor of long-term success, particularly following epicardial ablation.23,28 It is noteworthy however that the inducibility of VT also depends on additional factors, including anesthesia, anti-arrhythmic drugs, and stimulation protocol, which may sometimes render its predictive value rather inconsistent.43
The benefit of VT ablation in ICM and NICM patients is unequivocal.15,17 The differences we report in recurrence between ICM and NICM will further inform both physicians and patients of the likelihood of ablation success and reinforce the need for tailored decisions. Nevertheless, future studies are necessary to test further strategies to optimize postprocedural outcomes, including systematical pre-ablation imaging protocols, complete endocardial and/or epicardial substrate homogenization and new techniques, namely pulsed-field ablation.11,12,46
LimitationsSeveral limitations must be acknowledged. Firstly, this was a retrospective single-center study including a rather small sample size. There is no information regarding changes in the anti-arrhythmic or HF medication during follow-up, which may also have introduced confounding. Thirdly, ICM and NICM populations have multiple distinct clinical characteristics, and although we have tried to overcome such limitation by adopting a propensity-score matched analysis, there are likely other competing risk factors for the study endpoints that were not considered in the model. It was not possible to collect information on the characteristics of ventricular scars (voltage maps or CMR) in all patients, and pre-procedure imaging was not performed systematically. Finally, as mortality was ascertained by means of a national registry, we could not distinguish between arrhythmic and non-arrhythmic causes of death. In the more advanced stages of HF, arrhythmic events are a marker of severity, and its treatment may not affect overall survival.42
ConclusionNon-ischemic cardiomyopathy patients are at increased risk of VT recurrence after ablation, although long-term mortality was similar to ICM in a propensity-matched cohort. Non-inducibility of any VT after CA is associated with better outcomes.
Conflicts of interestThe authors have no conflicts of interest to declare.