Isolated left ventricular noncompaction (ILVNC) is a rare cardiomyopathy characterized by a prominent trabecular meshwork and deep intertrabecular recesses. The present study aimed to examine right atrial (RA) volumes, volume-based functional properties and strains by three-dimensional speckle-tracking echocardiography (3DSTE) in ILVNC patients.
MethodsThe study group consisted of 13 patients with ILVNC (mean age: 54.6±14.1 years, six male) and 23 healthy age- and gender-matched volunteers (mean age: 50.4±12.4 years, 10 male), who served as normal controls. Complete two-dimensional Doppler echocardiography and 3DSTE were performed in all cases.
ResultsIncreased systolic maximum (58.2±15.3 ml vs. 40.5±11.8 ml, p=0.0004) and diastolic pre-atrial contraction (39.6±16.1 ml vs. 28.2±9.2 ml, p=0.01) and minimum (46.2±17.5 ml vs. 34.6±11.6 ml, p=0.02) RA volumes were detected in ILVNC patients. Only total (18.6±8.5 ml vs. 12.2±5.9 ml, p=0.01) and passive (12.0±13.3 vs. 5.9±3.7 ml, p=0.05) RA stroke volumes, representing features of RA reservoir and conduit phases, were increased in ILVNC; active RA stroke volume and all emptying fractions did not differ between ILVNC patients and matched controls. Moreover, global, mean segmental and regional peak strains and strains at atrial contraction showed no differences between ILVNC patients and controls.
Conclusions3DSTE-derived volumetric analysis confirmed increased cyclic RA volumes in ILVNC. Only mild RA functional alterations were demonstrated in ILVNC.
A miocardiopatia não compactada isolada do ventrículo esquerdo (NCIVE) foi apresentada como uma miocardiopatia rara, caracterizada por uma trabeculação proeminente e por profundos recessos trabeculares na cavidade ventricular. Este estudo tem como objetivo examinar os volumes da aurícula direita (AD) e a mecânica auricular direita por ecocardiografia tridimensional de speckle-tracking nos doentes com NCIVE.
MétodosO grupo de estudo é composto por 13 doentes com NCIVE (idade média: 54,6 ± 14,1 anos, seis homens) e 23 voluntários saudáveis com idade e género correspondentes (idade média: 50,4 ± 12,4 anos, homens) que são controlos normais. Foram realizadas em todos os casos ecocardiografia Doppler bidimensional e ecocardiografia tridimensional de speckle-tracking.
ResultadosFoi detetado nos doentes com NCIVE um aumento do volume da AD na fase sistólica (58,2 ± 15,3 ml versus 40,5 ± 11,8 ml, p = 0,0004), na fase de contração diastólica pré-auricular (39,6 ± 16,1 ml versus 28,2 ± 9,2 ml, p = 0,01) e na fase diastólica final (46,2 ± 17,5 ml versus 34,6 ± 11,6 ml, p = 0,02). Apenas o volume da fase de reservatório (18,6 ± 8,5 ml versus 12,2 ± 5,9 ml, p = 0,01) e da fase de condução da AD (12,0 ± 13,3 versus 5,9 ± 3,7 ml, p = 0,05) foram superiores no grupo NCIVE em comparação com o grupo de controlos. O volume de ejeção da aurícula direita foi semelhante entre ambos os grupos. Os grupos foram também homogéneos relativamente ao strain e ao strain rate da AD nas diferentes fases do ciclo cardíaco.
ConclusãoA análise volumétrica detetada pela ecocardiografia tridimensional de speckle-tracking confirmou o aumento cíclico dos volumes da AD na NCIVE. Apenas alterações funcionais suaves da AD podem ser demonstradas na NCIVE.
Isolated left ventricular noncompaction (ILVNC) is a rare cardiomyopathy characterized by a prominent trabecular meshwork and deep intertrabecular recesses.1–3 The disorder seems to occur because of an arrest of the normal compaction process in myocardial development during the first trimester. The classic triad of ILVNC-related complications are heart failure, arrhythmias, and systemic embolic events. Although the usual site of involvement is the left ventricle (LV), the right ventricle (or both) are also affected in some cases.4,5 Due to atrioventricular interactions, left atrial (LA) function may show alterations, as has been demonstrated in ILVNC.6 However, no clinical studies on right atrial (RA) function have been performed in series of patients with ILVNC. Therefore, the present study aimed to examine RA volumes, volume-based functional properties and strains by three-dimensional (3D) speckle-tracking echocardiography (STE) in ILVNC patients.
MethodsStudy populationThe study population consisted of 13 patients with ILVNC from the Cardiology Center of the University of Szeged, Hungary, and 23 age- and gender-matched healthy volunteers, who served as normal controls. All patients and controls were in sinus rhythm and all were examined by two-dimensional Doppler echocardiography and 3DSTE. The echocardiographic diagnostic criteria for ILVNC of Jenni et al.1 were used:
- (1)
Segmental and excessive thickening of the LV wall with a two-layered structure, consisting of a thin, compacted epicardial layer and a thicker, noncompacted layer. The latter has a characteristic appearance with numerous prominent trabeculations (meshwork) and deep intertrabecular recesses. LV thickening is predominant in the apical, mid-lateral, and mid-inferior walls.
- (2)
A noncompacted/compacted myocardial thickness ratio >2 measured at maximal thickness in end-systole in parasternal short-axis view.
- (3)
Evidence of deeply perfused intertrabecular recesses communicating with the LV cavity by color Doppler echocardiography.
- (4)
Coexisting cardiac anomalies are absent.
The present work is part of the Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases (MAGYAR-Path) Study (‘Magyar’ means ‘Hungarian’ in the Hungarian language), which aims to examine the diagnostic and prognostic significance of 3DSTE-derived parameters in specific disorders. The study protocol was approved by the institutional review board on biomedical research, and all patients and healthy subjects gave informed consent consistent with the protocol.
M-mode and two-dimensional Doppler echocardiographyA Toshiba Artida™ ultrasound system (Toshiba Medical Systems, Tokyo, Japan) with a PST-30SBP (1-5 MHz) phased-array transducer was used for standard Doppler echocardiographic examinations. All echocardiographic studies were digitally recorded and evaluated by experts (AK, DP). Simpson's method was used to calculate LV ejection fraction, while LV and LA internal dimensions were measured by M-mode echocardiography. Tricuspid annular plane systolic excursion (TAPSE) and right ventricular fractional area change (RVFAC) were calculated to characterize RV function. Color Doppler echocardiography was used to visually quantify the degree of tricuspid and mitral regurgitation.
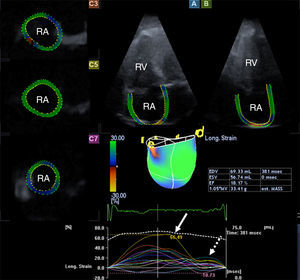
Three-dimensional speckle tracking echocardiographyA special matrix phased-array transducer (2.5 MHz PST-25SX; Toshiba Medical Systems, Tokyo, Japan) was used for the acquisition of 3D echocardiographic datasets in apical view.6,7 Six R-wave-triggered subvolumes were acquired during six consecutive cardiac cycles and one breath-hold to form the pyramid-shaped 3D full volume including the RA. 3D Wall Motion Tracking software version 2.7 (Toshiba Medical Systems, Tokyo, Japan) was used for RA quantifications. Firstly, apical two-chamber (AP2CH) and four-chamber (AP4CH) views and three short-axis views at different levels of the RA at end-diastole were automatically selected from the full volume 3D dataset by the software. After finding optimal non-foreshortened views by optimizing long-axis views, the reader set markers on orthogonal AP2CH and AP4CH views. Firstly, the edge of the lateral wall-tricuspid annulus was traced, then markers were set in a counterclockwise rotation around the RA to the edge of the septum-tricuspid annulus. The RA appendage, caval veins and coronary sinus were excluded from the RA cavity during evaluations. Finally, the 3D endocardial surface of the RA was automatically reconstructed and tracked in 3D space throughout the entire cardiac cycle. The user could correct the shape of the RA if needed (Figure 1). The same 3DSTE-derived methodology for detailed assessment of atrial function including volumetric and strain measurements applied initially for the LA was used during evaluations.8
Images from the three-dimensional speckle-tracking echocardiography-derived full-volume dataset. Apical 4-chamber (A) and 2-chamber (B) views and parasternal short-axis views at basal (C3), mid-atrial (C5) and superior (C7) right atrial level are displayed. Right atrial volumetric data and three-dimensional right atrial cast together with time-global volume (dashed line) and segmental time-longitudinal strain (colored lines) curves are also demonstrated. The method presented allows automatic measurement of peak strains (white arrow) and strains at atrial contraction (dashed arrow). EDV: end-diastolic volume; ESV: end-systolic volume; EF: ejection fraction; RA: right atrium; RV: right ventricle.
The following RA volumetric data were calculated: end-systolic maximum RA volume (Vmax, before tricuspid valve opening); early diastolic RA volume before atrial contraction (VpreA, at the time of the P wave on the ECG); and end-diastolic minimum RA volume (Vmin, before tricuspid valve closure). From RA cyclic volumetric data several parameters featuring all phases of LA function (reservoir, conduit and active contraction) were calculated (Table 1).10
Calculation of right atrial volume-based functional properties in each phase of atrial function.
| Reservoir | Conduit function | Active contraction | |
|---|---|---|---|
| Stroke (emptying) volumes (ml) | Total SV=Vmax-Vmin | Passive SV=Vmax-VpreA | Active SV=VpreA-Vmin |
| Emptying fractions (%) | Total EF=total SV/Vmax | Passive EF=passive SV/Vmax | Active EF=active SV/VpreA |
EF: emptying fraction; LV: left ventricular; SV: stroke volume; Vmax: maximum right atrial volume; Vmin: minimum right atrial volume; VpreA: right atrial volume before atrial contraction.
Using the same 3D dataset, similarly to other studies, several one-directional (longitudinal, LS; radial, RS; circumferential, CS) and complex (area strain: AS; 3D strain: 3DS) strain parameters were measured in each case.10 Peak strains were calculated for the RA reservoir phase, while strains at atrial contraction were calculated for the RA booster pump phase. Global, mean segmental and regional strains were measured.
Statistical analysisResults are expressed as mean ± standard deviation. Data were compared with the two-tailed Student's t test, chi-square analysis, and Fisher's exact test. Statistical significance was a p value <0.05. All calculations were performed with MedCalc software (MedCalc, Inc., Mariakerke, Belgium).
ResultsClinical and two-dimensional echocardiographic dataClinical and two-dimensional echocardiographic features of ILVNC patients and of controls are presented in Table 2. Significant (grade >2) mitral and tricuspid regurgitation was detected in four (31%) and two (15%) patients with ILVNC, respectively, but in none of the controls. LV dimensions were significantly increased in ILVNC, while LV ejection fraction was significantly reduced (Table 2). The TAPSE and RVFAC values of ILVNC patients were 14.8±4.3 mm and 35.5±3.2%, respectively. Five ILVNC patients had reduced RVFAC (mean 31.8±0.84%), while eight ILVNC patients had normal RVFAC (mean 37.8±1.0%).
Clinical and two-dimensional echocardiographic features of patients with isolated left ventricular noncompaction and of controls.
| ILVNC patients (n=13) | Controls (n=23) | p | |
|---|---|---|---|
| Risk factors | |||
| Age (years) | 54.6±14.1 | 50.4±12.4 | 0.54 |
| Male gender (%) | 6 (46) | 10 (43) | 0.72 |
| Diabetes mellitus (%) | 0 (0) | 0 (0) | 1.00 |
| Hypertension (%) | 5 (39) | 0 (0) | 0.004 |
| Hypercholesterolemia (%) | 3 (23) | 0 (0) | 0.04 |
| Medication | |||
| Beta-blockers (%) | 10 (77) | 0 (0) | <0.0001 |
| ACE inhibitors (%) | 10 (77) | 0 (0) | <0.0001 |
| Diuretics (%) | 9 (69) | 0 (0) | <0.0001 |
| Two-dimensional echocardiography | |||
| LA diameter (mm) | 46.7±9.5 | 34.0±3.3 | <0.0001 |
| LV end-diastolic diameter (mm) | 63.6±12.4 | 47.7±8.5 | 0.0001 |
| LV end-diastolic volume (ml) | 204.2±85.1 | 101.3±33.9 | <0.0001 |
| LV end-systolic diameter (mm) | 48.6±14.5 | 29.2±4.4 | <0.0001 |
| LV end-systolic volume (ml) | 121.2±72.4 | 33.2±11.0 | <0.0001 |
| Interventricular septum (mm) | 10.3±1.8 | 9.6±1.7 | 0.24 |
| LV posterior wall (mm) | 10.0±1.1 | 9.4±2.0 | 0.33 |
| LV ejection fraction (%) | 38.6±15.6 | 66.4±6.8 | <0.0001 |
| E/A | 1.7±0.6 | 1.3±0.1 | 0.008 |
| Number of noncompacted segments | 6.7±1.3 | 0 | - |
ACE: angiotensin-converting enzyme; ILVNC: isolated left ventricular noncompaction; LA: left atrium; LV: left ventricular.
The mean volume rate was 27±3 Hz. Increased Vmax, VpreA and Vmin were detected in ILVNC patients (Table 3). Only total (TASV) and passive (PASV) RA stroke volumes, representing features of the RA reservoir and conduit phases, were found to be increased in ILVNC; active RA stroke volume (AASV) and all emptying fractions did not differ between ILVNC patients and matched controls. Global, mean segmental and mean regional peak strains and strains at atrial contraction did not show significant differences between ILVNC patients and controls (Tables 4–7). Increased Vmax (59.0±16.9 ml vs. 58.1±12.6 ml, p=0.92), Vmin (38.7±16.0 ml vs. 43.2±14.8 ml, p=0.62), VpreA (45.0±18.4 ml vs. 51.0±12.9 ml, p=0.54), TASV (20.3±9.5 ml vs. 14.9±5.4 ml, p=0.28) and PASV (14.9±15.4 ml vs. 7.1±3.5 ml, p=0.28) were observed in ILVNC patients regardless of the presence or absence of RV dysfunction demonstrated by RVFAC.
Comparison of three-dimensional speckle-tracking echocardiography-derived right atrial volumes and volume-based functional properties in patients with isolated left ventricular noncompaction and in controls.
| ILVNC patients (n=13) | Controls (n=23) | p | |
|---|---|---|---|
| Calculated volumes | |||
| Vmax (ml) | 58.2±15.3 | 40.5±11.8 | 0.0004 |
| Vmin (ml) | 39.6±16.1 | 28.2±9.2 | 0.01 |
| VpreA (ml) | 46.2±17.5 | 34.6±11.6 | 0.02 |
| Stroke volumes | |||
| TASV (ml) | 18.6±8.5 | 12.2±5.9 | 0.01 |
| PASV (ml) | 12.0±13.3 | 5.9±3.7 | 0.05 |
| AASV (ml) | 6.6±7.3 | 6.4±4.6 | 0.91 |
| Emptying fractions | |||
| TAEF (%) | 33.5±14.7 | 30.5±10.2 | 0.49 |
| PAEF (%) | 20.5±20.6 | 14.9±8.6 | 0.26 |
| AAEF (%) | 12.3±23.9 | 18.2±9.4 | 0.30 |
AAEF: active atrial emptying fraction; AASV: active atrial stroke volume; FR: frame rate; PAEF: passive atrial emptying fraction; PASV: passive atrial stroke volume; TAEF: total atrial emptying fraction; TASV: total atrial stroke volume; Vmax: maximum left atrial volume; Vmin: minimum left atrial volume; VpreA: volume before atrial contraction.
Comparison of three-dimensional speckle-tracking echocardiography-derived peak global and mean segmental right atrial strain parameters in patients with isolated left ventricular noncompaction and in controls.
| ILVNC patients (n=13) | Controls (n=23) | p | |
|---|---|---|---|
| Peak global strain | |||
| RS (%) | −10.1±7.8 | −15.0±9.1 | 0.11 |
| CS (%) | 13.2±14.8 | 9.5±8.3 | 0.35 |
| LS (%) | 22.9±14.4 | 23.0±10.3 | 0.98 |
| 3DS (%) | −5.5±5.9 | −7.8±5.7 | 0.27 |
| AS (%) | 39.5±38.8 | 30.9±17.6 | 0.36 |
| Peak mean segmental strain | |||
| RS (%) | −14.0±7.2 | −18.7±8.0 | 0.09 |
| CS (%) | 18.3±14.6 | 15.0±8.3 | 0.40 |
| LS (%) | 25.7±13.7 | 26.9±9.5 | 0.77 |
| 3DS (%) | −8.9±6.2 | −12.4±5.9 | 0.10 |
| AS (%) | 46.2±38.1 | 38.7±16.7 | 0.41 |
3DS: three-dimensional strain; AS: area strain; CS: circumferential strain; ILVNC: isolated left ventricular noncompaction; LS: longitudinal strain; RS: radial strain.
Comparison of three-dimensional speckle-tracking echocardiography-derived peak mean regional right atrial strain parameters in patients with noncompaction cardiomyopathy and in controls.
| ILVNC patients (n=13) | Controls (n=23) | p | |
|---|---|---|---|
| RS basal (%) | −13.4±6.7 | −17.4±6.4 | 0.09 |
| RS mid (%) | −14.4±7.5 | −18.6±9.6 | 0.18 |
| RS apical (%) | 14.4±10.8 | −20.7±12.0 | 0.13 |
| CS basal (%) | 15.8±12.9 | 18.0±11.0 | 0.59 |
| CS mid (%) | 15.1±12.6 | 13.0±7.4 | 0.54 |
| CS apical (%) | 22.2±21.0 | 13.2±15.2 | 0.15 |
| LS basal (%) | 26.7±14.1 | 25.0±12.0 | 0.70 |
| LS mid (%) | 30.8±17.7 | 37.9±15.9 | 0.22 |
| LS apical (%) | 15.5±14.3 | 13.3±8.0 | 0.56 |
| 3DS basal (%) | −8.5±5.8 | −11.6±5.6 | 0.14 |
| 3DS mid (%) | −8.7±6.2 | −11.4±6.5 | 0.23 |
| 3DS apical (%) | −9.7±8.9 | −15.0±10.6 | 0.14 |
| AS basal (%) | 40.5±30.6 | 35.0±16.9 | 0.49 |
| AS mid (%) | 48.2±34.8 | 48.0±20.6 | 0.98 |
| AS apical (%) | 51.6±61.7 | 30.4±34.9 | 0.19 |
3DS: three-dimensional strain; AS: area strain; CS: circumferential strain; ILVNC: isolated left ventricular noncompaction; LS: longitudinal strain; RS: radial strain.
Comparison of three-dimensional speckle-tracking echocardiography-derived global and mean segmental right atrial strains at atrial contraction in patients with isolated left ventricular noncompaction and in controls.
| ILVNC patients (n=13) | Controls (n=23) | p | |
|---|---|---|---|
| Global strain at atrial contraction | |||
| RS (%) | −6.1±6.7 | −6.7±6.3 | 0.77 |
| CS (%) | 5.6±6.0 | 8.9±10.9 | 0.32 |
| LS (%) | 9.4±10.9 | 8.8±7.9 | 0.84 |
| 3DS (%) | −4.6±5.0 | −4.2±4.6 | 0.80 |
| AS (%) | 12.5±10.9 | 15.2±14.3 | 0.56 |
| Mean segmental strain at atrial contraction | |||
| RS (%) | −7.9±5.3 | −8.9±4.6 | 0.54 |
| CS (%) | 7.6±6.8 | 11.3±10.1 | 0.25 |
| LS (%) | 9.9±8.5 | 8.4±5.6 | 0.53 |
| 3DS (%) | −5.8±5.1 | −6.8±4.4 | 0.51 |
| AS (%) | 18.1±16.0 | 19.0±14.7 | 0.86 |
3DS: three-dimensional strain; AS: area strain; CS: circumferential strain; ILVNC: isolated left ventricular noncompaction; LS: longitudinal strain; RS: radial strain.
Comparison of three-dimensional speckle-tracking echocardiography-derived mean regional right atrial strains at atrial contraction in patients with isolated left ventricular noncompaction and in controls.
| ILVNC patients (n=13) | Controls (n=23) | p | |
|---|---|---|---|
| RS basal (%) | −7.64±5.8 | −9.9±5.7 | 0.26 |
| RS mid (%) | −7.9±5.8 | −8.1±5.2 | 0.94 |
| RS apical (%) | −8.4±6.4 | −8.7±5.3 | 0.88 |
| CS basal (%) | 7.3±7.5 | 13.2±11.0 | 0.10 |
| CS mid (%) | 7.2±7.0 | 9.7±8.6 | 0.37 |
| CS apical (%) | 8.8±8.2 | 8.5±13.4 | 0.96 |
| LS basal (%) | 8.5±8.9 | 6.8±6.1 | 0.49 |
| LS mid (%) | 12.8±10.4 | 10.7±7.8 | 0.49 |
| LS apical (%) | 10.2±8.9 | 7.8±9.3 | 0.46 |
| 3DS basal (%) | −4.8±4.7 | −7.7±5.4 | 0.12 |
| 3DS mid (%) | −6.1±5.5 | −6.0±4.2 | 0.93 |
| 3DS apical (%) | −6.8±7.4 | −6.7±5.1 | 0.96 |
| AS basal (%) | 12.3±15.5 | 17.1±11.0 | 0.29 |
| AS mid (%) | 20.3±17.5 | 21.7±14.0 | 0.79 |
| AS apical (%) | 19.8±17.3 | 18.0±29.7 | 0.84 |
3DS: three-dimensional strain; AS: area strain; CS: circumferential strain; ILVNC: isolated left ventricular noncompaction; LS: longitudinal strain; RS: radial strain.
In recent studies it has been suggested that ILVNC could be part of a more widespread cardiomyopathy.2 Significant alterations in LV and LA dimensions and functional properties have been demonstrated in ILVNC.6,11–18 However, the degree of the functional involvement of the right heart needs to be clarified. It is also important to understand whether alterations are related directly to LV dysfunction, or whether other factors are in play. To the best of the authors’ knowledge this is the first detailed analysis of RA morphology and function in ILVNC. Although RV involvement could not be confirmed in any of the cases, increased RA volumes and RA stroke volumes characterizing RA reservoir and conduit phases were detected in ILVNC patients. Neither RA emptying fractions nor RA strains showed differences between ILVNC patients and matched controls, suggesting only slight alterations in RA function. RA dysfunction was not confined to ILVNC patients with RV dysfunction, but was also seen in patients with normal RV function.
At present little is known about myocardial mechanics in ILVNC, due to its rarity and the lack of large ILVNC registries. Modern echocardiographic methodologies including STE and/or 3D echocardiography have been demonstrated to be more useful for quantitative objective analysis of ventricular and atrial function than conventional techniques, including in ILVNC.6,11–20 In a recent real-time 3D echocardiography (RT3DE) study, noncompacted and compacted LV segments had comparable increased 3D regional volumes and reduced systolic function.11 In another study, all 3DSTE-derived LV strains of segments in ILVNC patients were found to be significantly reduced, and this strain reduction was not confined to noncompacted segments,12 with non-compacted segment-related RS and 3DS reductions more pronounced than in compacted segments. Ari et al. found that tissue Doppler imaging- and STE-derived myocardial deformation can be used for the detection of early myocardial dysfunction in patients with subclinical ILVNC whose LV function was classified as normal by conventional methods, with normal ejection and shortening fraction.13 As well as the above-mentioned alterations in quantitative features of LV wall motion abnormalities, LV ‘rigid body rotation’, the near absence of LV twist, was found to be a frequent phenomenon in ILVNC as well.15,16
Besides these LV studies, atrial morphology and function in ILVNC patients have been examined in very few studies.6,17,18 Given the value of 3DSTE for volumetric and strain analysis of all atrial functions including systolic reservoir and diastolic conduit and booster pump phases simultaneously, a study was planned focusing on LA function at our center.6 Increases in LA volumes and decreases in LA emptying fractions, together with reductions in all LA peak strains, suggested significant deterioration of all LA functions.6 In another study increased LA ejection force, a characteristic of atrial contraction, was demonstrated in ILVNC in a 3DSTE study,18 which suggested increased LA work to compensate for the dysfunctional LV.
Despite its technical limitations, 2D Doppler echocardiography is the standard method for assessing the RA in everyday clinical practice and magnetic resonance imaging is the current gold standard for RA visualization and volume quantification.21 Real-time three-dimensional echocardiography (RT3DE) was introduced over a decade ago for non-invasive quantification of the heart chambers including the RA.19,20 In a recent publication, normal values of RT3DE-derived RA volumes and volume-based functional properties were established.20 To find the optimal RT3DE method for assessing RA volumes, another RT3DE study was performed in which multiple planes were used for volumetric quantifications. Four-plane measurement of RA volume using four equiangular planes showed good agreement with eight-plane measurement, while reducing the time required for analysis. However, compared to eight-plane analysis, biplane measurement of RA volume can result in underestimation of RA volume, particularly in abnormal subjects with RA enlargement and remodeling.19
In the present study, RA function was characterized by the same 3DSTE methodology as demonstrated previously for the LA.6,10 3DSTE was found to be feasible for estimation of RA function.22,23 In a recent work, significant alterations in LA volume-based and strain characteristics were demonstrated in ILVNC, as mentioned above.6 By contrast, only TASV and PASV were found to be increased, suggesting mild RA functional alterations in this study. Although only a small number of ILVNC patients were examined, the impact of LV dysfunction due to the arrest of the LV compaction process on atrial function is demonstrated indirectly. Alterations in RA functional properties were present regardless of the presence or absence of RV dysfunction. Nevertheless, only ILVNC patients had tricuspid (and mitral) regurgitation, and thus the associated volume overload may have affected the results obtained. Moreover, LA-RA interactions should also be taken into consideration, since the functional properties of the atrial septum were also relevant to the findings. However, further studies are warranted with larger ILVNC patient populations to confirm our findings.
LimitationsThe study has several limitations:
- -
The RA appendage, coronary sinus and caval veins were excluded from assessments, as in other 3DSTE studies assessing the RA.22,23 This should be taken into consideration when interpreting the results.
- -
Although there was the opportunity to assess the LV, RV and LA by 3DSTE, this was beyond the scope of this study.
- -
Only a small number of ILVNC patients from a single tertiary center were examined. However, it should be borne in mind that ILVNC is a relatively rare disorder. Therefore, further multicenter 3DSTE studies with larger patient populations are warranted to confirm our findings.
- -
There were important technical limitations, including low spatial and temporal resolution.7–9 It is known that 3DSTE is currently subject to these technical limitations, and significant improvements in image resolution are required.
- -
Finally, there is disagreement as to whether the atrial septum is a part of the LA or the RA. In the present study the septum was considered as a part of the virtual 3D cast of the RA.
3DSTE-derived volumetric analysis confirmed increased cyclic RA volumes in ILVNC. Only mild RA functional alterations were demonstrated in ILVNC.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.