A 71-year-old man with Chagas disease and stable angina on minimum exertion underwent coronary computed tomography angiography and cine angiography that revealed heavily calcified multivessel disease involving the left main artery (LM). Due to the degree of calcification, it was decided to perform rotablation. The first-stage percutaneous coronary intervention (PCI) with rotablation was performed on the LM, left anterior descending artery and second diagonal branch without complications. Almost 30 days later he returned for right coronary artery (RCA) PCI. The proposed strategy was rotational atherectomy in the posterior descending artery (PDA) and right posterolateral artery (RPLA) with a 1.5 mm burr, followed by implantation of two drug-eluting stents (DES). Through right femoral artery access the RPLA lesion was ablated with success. As there were no signs of dissection and TIMI 3 flow was maintained, the 0.009″ RotaWire was repositioned to cross the PDA lesion and debulking of the lesion was performed. After two attempts we succeeded in crossing the lesion with the 1.5 mm burr, however entrapment of the burr ensued. The system was pulled back until the guiding catheter penetrated deep into the RCA, and attempts were made to release the Rotablator by moving it forward and backward, but the burr did not even spin. The contralateral femoral artery was therefore punctured and a 6F JR guiding catheter was inserted, in order to move a guidewire and small angioplasty balloon tangentially to the burr, but without success. Finally we advanced the guidewire using the ‘knuckle’ technique, taking advantage of the kinking of the distal portion of the PT2 guidewire, performing a subintimal dissection and re-entry, and could then easily cross the balloon, inflate it and release the trapped burr. Through the 6F system, two programmed and one bailout DES were successfully implanted in the PDA, RPLA and RCA, obtaining final TIMI 3 flow without complications.
Um homem de 71 anos, diabético, com doença de Chagas e com angina estável de mínimos esforços, efetuou angiotomografia coronária e cineangiocoronariografia revelando doença multiarterial severamente calcificada envolvendo o tronco da coronária esquerda (TCE). Devido ao grau de calcificação, a aterectomia rotacional foi considerada. Na primeira etapa a angioplastia coronária (ATC) com aterectomia rotacional (Rotablator) foi realizada no TCE, artéria descendente anterior e segundo ramo diagonal sem intercorrências. Quase 30 dias depois retornou para ATC da artéria coronária direita (CD). A estratégia proposta foi a aterectomia rotacional nos ramos DP e VPD com oliva de 1,5 mm, seguida de implante de dois stents farmacológicos (DES). Por acesso femoral direito 7F, a lesão do VPD foi ablacionada com sucesso. Como não havia sinais de disseção e manutenção de fluxo TIMI III, reposicionamos o “Rotawire 0,009” atravessando a lesão do DP e procedemos à ablação da lesão. Depois de duas tentativas conseguimos cruzar a lesão; no entanto, houve o aprisionamento da oliva. O sistema foi tracionado sem sucesso, levando o catéter guia a penetrar fundo na CD, com posterior disseção. Foi tentada a retirada do “Rotablator” com movimentos de avanço e recuo, mas a oliva sequer girou. A decisão então foi puncionarmos a artéria femoral contralateral inserindo um cateter guia JR6F e tentarmos avançar uma corda guia e balão de fino calibre tangencialmente à oliva. Não obtivemos sucesso até que finalmente avançamos o fio-guia com a técnica de Knuckle aproveitando a dobra da extremidade distal do fio-guia PT2 e pudemos facilmente atravessar o balão, insuflar e libertar o aprisionamento da oliva. Através do catéter guia 6F, os dois stents programados (DP e VPD) e um adicional (CD) foram implantados com sucesso obtendo-se fluxo final TIMI III, sem intercorrências clínicas.
Heavily calcified lesions in percutaneous coronary intervention (PCI) require the use of rotational atherectomy (RA) in order to improve stent deliverability and avoid incomplete stent expansion and malapposition, which may consequently predispose to in-stent restenosis or thrombosis.1,2 The use of RA is not without risks. One of the major and most feared complications, although rare (reported incidence of 0.4%),3 is burr entrapment, which can lead to serious consequences including fatal arrhythmias and myocardial ischemia or infarction due to intracoronary thrombosis. In such cases, the patient usually undergoes an emergency surgical bypass procedure, which can further increase mortality and morbidity.4
Here we report a case of burr entrapment and discuss the management of this complication using the ‘knuckle’ technique, an innovative and unorthodox method usually adopted in chronic total occlusions (CTO), aiming to avoid an emergency open chest procedure.
Case reportA 71-year-old man with diabetes and Chagas disease had recent significant weight loss (60 kg). The clinical investigation resulted in a diagnosis of megaesophagus associated with significant obstruction at the level of the cardia. Esophageal balloon dilatation was performed, successfully recovering digestive transit. In the meantime, during surgical risk assessment, the patient also presented chest pain on minimum exertion. Coronary computed tomography angiography performed three years earlier showed heavily calcified multivessel disease involving the left main (LM); no further investigation had been performed at that time. This time he was referred for us for cardiac catheterization.
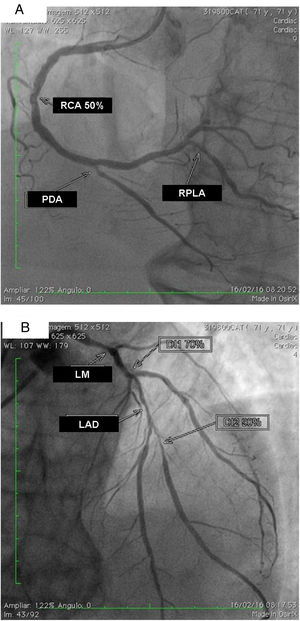
The exam carried out on 16 February, 2016 showed: right coronary artery (RCA) with important calcification and a 50% lesion in the mid third; posterior descending artery (PDA) and right posterolateral artery (RPLA), with severe calcification and lesions of 80% and 70%, respectively (Figure 1A); LM with 80% calcified lesions in the distal third; left anterior descending artery (LAD) with significant calcification and 90% lesion in the mid third; diagonal branches (DG1 and DG2) with calcified lesions of 70% and 80%, at the origin and in the proximal third, respectively; and left circumflex artery (LCx) occluded and calcified, receiving grade II collaterals of multiple origin (Figure 1B). Left ventricular volume and contractility were preserved.
(A) Right coronary artery (RCA) with important calcification and 50% lesion in the mid third, posterior descending artery (PDA) and right posterolateral artery (RPLA) with severe calcification and lesions of 80% and 70%; respectively; (B) left main (LM) with calcified lesions of 80% in the distal third, left anterior descending artery (LAD) with significant calcification and 90% in the mid third, and diagonal branches (DG1 and DG2) with calcified lesions of 70 and 80%, respectively. The left circumflex is occluded and calcified.
With this angiographic picture, Society of Thoracic Surgeons risk score 15.8% for morbidity or mortality, EuroSCORE II 4.67%, and significant frailty due to recent major weight loss, the heart team decided to perform percutaneous coronary intervention (PCI) of the LM, LAD, DG1 and DG2 initially and, in a second procedure after 30 days, PCI of the RCA branches. In both procedures, RA was also indicated due to significant calcification. The LCx would not be addressed since angiographically the vessel was not so severely involved and also considering the technical difficulty of recanalization, due to the length of the CTO and also heavily calcified walls (J-CTO score 4). The LCx was already receiving grade II collateral circulation.
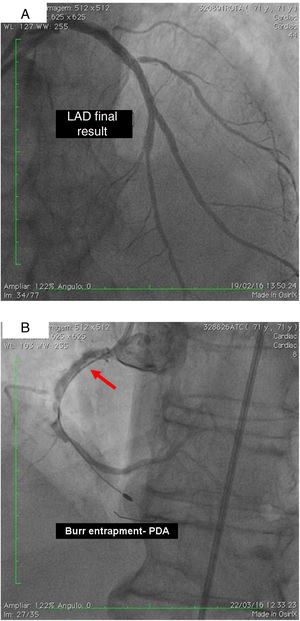
On 19 February, 2016, after beginning dual antiplatelet therapy with aspirin and clopidogrel, the patient underwent RA of the LM, LAD and DG2 with a 1.5 mm burr followed by predilation with a 3.0 mm×20 mm balloon at 14 atm and implantation of drug-eluting stents (DES) in DG2 and LAD, using a mini-crush technique and a kissing balloon at the end. A kissing balloon was then performed at the LAD/DG1 bifurcation and finally the third DES was implanted from the origin of the LM to overlap with the LAD stent. Procedural success was achieved with TIMI flow 3 and without clinical or angiographic complications (Figure 1B).
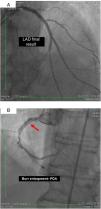
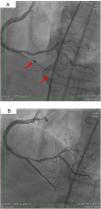
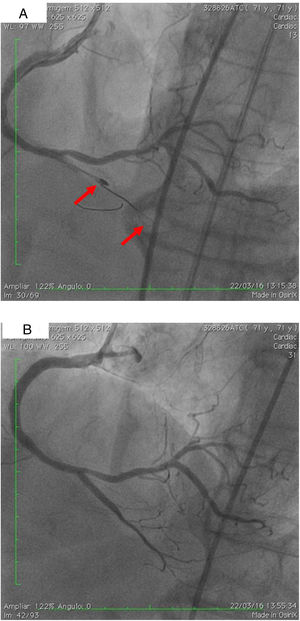
On 22 March, 2016, the patient returned for RCA PCI (Figure 2A). The proposed strategy was RA in the PDA and RPLA with a 1.5 mm burr, followed by implantation of two DES. Through right femoral access and a 7F JR guide catheter we crossed the RotaWire extra support through the RPLA lesion, then conducted three successive 20-s passes at 170 000 rpm successfully and uneventfully. As there were no signs of dissection and TIMI 3 flow was maintained, the 0.009″ RotaWire was repositioned to cross the PDA lesion and debulking of the lesion was performed. After two attempts with short, gentle movements we succeeded in crossing the lesion, however entrapment of the burr ensued (Figure 2B). The system was pulled back without success considering that the stretching of the system was causing wrinkles at the RCA and the 7F JR was penetrating deep into the artery, with risk of rupture. Attempts were then made to release the Rotablator, in both rotablation and dynaglide modes, by moving it forward and backward, but the burr did not even spin. Attempts were made to advance a parallel guidewire with 1.25 mm balloon but it did not progress within the 7F guide catheter. We therefore decided to puncture the left (contralateral) femoral artery and insert a 6F JR guide catheter to the RCA ostium, parallel to the first 7F guide catheter, and advance a PT2® guidewire (Boston Scientific) to the PDA branch with a 1.25 mm×8 mm balloon upstream to support this wire. We succeeded in arriving parallel to the Rotablator system until the proximal portion of the entrapment site. Attempts to cross the guidewire and balloon angioplasty tangentially to the burr were unsuccessful because the tip of the wire was not sufficiently stiff to cross it. When the PT2 was pushed with the back support of the balloon this forceful movement caused the tip of the wire to bend, and finally the guidewire was advanced with the ‘knuckle’ technique (used for recanalization of CTOs), taking advantage of the kinking of the distal portion of the PT2 guidewire, making a subintimal dissection. At this time we could not see if the wire had re-entered the true lumen, but the 1.5 mm balloon could then easily be crossed parallel to the burr and inflated to 16 atm to release the trapped burr (Figure 3A). The burr was subsequently withdrawn maintaining the RotaWire in the initial position. After administration of 100 μg nitroglycerin, angiography confirmed that the PT2 guidewire had re-entered the true lumen and reached the most distal part of the PDA. The RotaWire was pulled out together with the whole 7F system. Through the 6F system and the same PT2 wire, PCI was finalized with implantation of the two programmed 2.75 mm×20 mm and 2.75 mm×16 mm DES at 12 atm in the PDA and RPLA, respectively, without any further predilation. We also noted the presence of a long and severe dissection in the mid third of the RCA, certainly caused by excessive handling and attempts to remove the burr, which caused deep penetration by the 7F guide catheter. This dissection was promptly corrected with implantation of a third 4.0 mm×32 mm DES, and final TIMI 3 flow was obtained without clinical or electrocardiographic complications (Figure 3B). The two femoral puncture sites were occluded with 8F and 6F AngioSeal devices, respectively. The patient remained in the intensive care unit for 48 hours, the only abnormality being CK-MB elevation (twice the reference value). He was discharged on day 3 in excellent general condition. The control echocardiogram showed normal left ventricular contractility.
(A) Percutaneous coronary intervention of the left main and left anterior descending artery (LAD) with two overlapping drug-eluting stents (DES) and the second diagonal branch with another mini-crush DES after rotablation with 1.5 mm burr, and ultimate success (TIMI flow 3); (B) burr entrapment in the posterior descending artery (PDA). The stiffness of the system when pullback was attempted is evident (arrow).
(A) Two parallel wires and the ‘knuckle’ at the distal lumen of the posterior descending artery (PDA) (arrow), with the 1.25 mm balloon tangential to the burr; (B) right coronary artery, PDA and right posterolateral artery, final result after implantation of three drug-eluting stents. Some improvement of collaterals to the left circumflex artery can be seen.
RA is a valuable tool to enable PCI in complex lesions with moderate or severe calcification when the heart team judges PCI to be appropriate. It should be available in the toolbox of every interventionalist who aims to deal with complex and high-risk patients. However, in recent series, RA use has fallen to 3-5% in select major centers and less than 1% in others.5 Though less often used nowadays, RA is still of use in the cardiac cath lab. The management of stable or unstable ischemic heart disease, particularly concerning the delivery of DES, is often hampered by calcification of plaques, and in such situations RA can be considered in order to improve conditions for the procedure itself, since superior success rates have been demonstrated when dealing with such complex calcified lesions.6 According to the European guidelines on myocardial revascularization,7 rotablation is recommended for preparation of heavily calcified or severely fibrotic lesions that cannot be crossed by a balloon or adequately dilated before planned stenting (class I recommendation), and the US guidelines8 have a similar recommendation (class IIa).
The use of RA is not without risks. Slow or no reflow, coronary spasm, distal embolization, coronary dissection or perforation, fracture of the guidewire or the drive shaft, and burr entrapment are often reported in the literature,9 although severe complications such as no or slow reflow, coronary perforation and shock are observed in fewer than 2% of procedures.6 Another problem is friction between the burr and plaque, which generates heat (2.6-13.9°C).10 Thermal injury can increase the risk of periprocedural myocardial infarction and restenosis. Modern techniques, favoring gradual, intermittent ablation with a pecking motion, aim to minimize deceleration and thermal injury.10
Kaneda et al. describe a situation in which a small burr can be advanced beyond a heavily calcified plaque before sufficient ablation, especially when the burr is pushed strongly at high rotational speed. They point out that at high-speed rotation, heat may enlarge the space between plaques, so that the burr can pass the calcified lesion easily without significant debulking of calcified tissue.3 Another situation can occur within a severely calcified long lesion, especially if angulated and with concomitant coronary spasm. If a large burr is used and pushed hard without appropriate pecking motions against such lesions, the rotational speed may decrease significantly and burr entrapment may occur.11
In summary, burr entrapment may happen when the burr passes to the distal portion of a lesion through an incompletely ablated segment. If the burr is advanced beyond a tight calcified lesion or embedded in a long, angulated and heavily calcified lesion, it can be trapped,7 as proximal movement is restricted by the absence of diamond chips on the back of the burr, prohibiting retrograde ablation.
In order to avoid this occurrence, the burr should never be allowed to stop spinning within a lesion. In addition, the operator should be attentive to warning signs, especially tactile, like resistance in the advancer knob or excessive drive shaft vibration. The use of smaller burrs and gradual, intermittent burr advancement may be useful to avoid entrapment.10 Entrapment of a rotablation burr is a rare but very serious complication of RA. Operators performing RA should be aware of this risk and be prepared to manage it appropriately.
Although urgent cardiac surgery is always an option when entrapment occurs, there is no doubt that the risks are increased in such a situation.9 Various percutaneous maneuvers can be performed to retrieve the entrapped burr, most of them involving applying the force of retraction as close to the site of entrapment as possible. These include firmly pulling back the whole system, using a ‘mother and child’ technique with a 5F catheter inside the 7F or 8F guide, or snaring and pulling the burr. Another option is to cross a second guide parallel to the burr and to advance small diameter balloons (1.5, 2.0 and 2.5 mm sequentially), aiming to inflate, enlarge the site of entrapment and consequently release the burr. This technique requires a new access site (usually contralateral) with a 6F guiding catheter via which the second wire and the small balloons can be advanced. Another possibility is to cut off the Rotablator system close to the advancer and remove the plastic sheath encircling the drive shaft, creating space to introduce a second coronary wire and small balloon through the same guiding catheter alongside the entrapped burr. Even with these maneuvers, it is often very hard to cross the site of the entrapped burr if it is tight and calcified.1,4,9 Knowing these obstacles, we had the idea to use CTO techniques to facilitate the transposition of a parallel extra support guidewire which would enable a balloon catheter to be advanced and inflated. In this case we chose the ‘knuckle’ technique, in which, to create a deliberate dissection plane, the wire, usually a polymer-jacketed guidewire, is pushed until a complex loop is formed and advanced through the lesion with no need for dedicated devices, reducing failure to cross the wire. In the case presented, the loop was easily able to negotiate around the proximal part of the burr and cross the point, reaching the true lumen of the vessel distally. There was then no difficulty in advancing and inflating the balloon and releasing the burr.
Conflicts of interestThe authors have no conflicts of interest to declare.