Hypertension is one of the most common chronic clinical problems encountered by physicians. The prevalence of resistant hypertension is estimated at 9% in the US. Patients with resistant hypertension have been shown to be at higher risk for adverse cardiovascular events, hence the need for greater efforts in improving the treatment of hypertension. The renal sympathetic nerves play an important role in the development of hypertension, mediated via sodium and water retention, increased renin release and alterations in renal blood flow. The proximity of the afferent and efferent renal sympathetic nerves to the adventitia of the renal arteries suggested the feasibility of an endovascular, selective, minimally invasive approach to renal denervation; a potential treatment option for resistant hypertension. While the RAPID, Reduce-HTN, EnligHTN, DENERHTN and Symplicity HTN-1 and -2 studies showed significant benefit of renal denervation in the treatment of resistant hypertension, the results of Oslo RDN, Prague-15 and Symplicity HTN-3 were not so favorable. Future well-designed clinical trials are needed to ascertain the benefits or otherwise of renal denervation in treatment-resistant hypertension.
A hipertensão arterial é um dos problemas clínicos crónicos mais frequentes. Nos EUA a prevalência de hipertensão arterial resistente está estimada em 9%. Os doentes com hipertensão resistente têm um maior risco de eventos cardiovasculares adversos, o que justifica maiores esforços na melhoria do tratamento da hipertensão. A inervação simpática renal tem um papel importante no desenvolvimento da hipertensão, mediada através da retenção de sódio e água, com aumento da libertação de renina e de alterações do fluxo sanguíneo renal. A anatomia da inervação simpática renal, com os seus nervos aferentes e eferentes em relação de proximidade com a adventícia, permite uma abordagem endovascular, seletiva e minimamente invasiva para a desnervação renal, e constitui uma opção de tratamento potencial para a hipertensão arterial resistente. Enquanto os estudos RAPID, Reduce-HTN, EngliHTN, DENERHTN e Simplicity HTN 1,2 mostraram um benefício significativo da desnervação renal no tratamento da hipertensão arterial resistente, os resultados dos estudos Oslo-RDN, Prague-15 e Simplicity HTN 3 não foram tão favoráveis. Serão necessários ensaios clínicos bem estruturados para confirmar ou infirmar os potenciais benefícios da desnervação renal no tratamento da hipertensão arterial resistente.
Hypertension is one of the most common chronic clinical problems encountered by physicians. Resistant hypertension is defined as systolic blood pressure (BP) ≥160 mmHg or (≥150 mmHg in patients with type 2 diabetes mellitus) refractory to medical treatment despite the use of optimal doses of three or more different drug types including a diuretic.1 The prevalence of resistant hypertension is estimated at 9% in the US.2 In a study of 205750 treated hypertensive patients, 1 in 50 developed resistant hypertension within a median of 1.5 years from the initial treatment.3 In the same study, patients with resistant hypertension were shown to be at higher risk for adverse cardiovascular events.3 The increased morbidity and mortality associated with this condition supports greater efforts to improve treatment options.
Historical perspectiveAfter a link was established between the sympathetic nervous system and the development of resistant hypertension, surgical sympathectomy was introduced in the 1930s, but was abandoned shortly afterwards due to multiple side effects such as postural hypotension, tachycardia, bladder, bowel and erectile dysfunction, and high periprocedural mortality.4 The proximity of the afferent and efferent renal sympathetic nerves to the adventitia of the renal arteries suggested the feasibility of an endovascular, selective, minimally invasive approach to renal denervation (RDN). In the endovascular approach, a treatment catheter is introduced through a guiding catheter into the renal arteries. The catheter is placed in close proximity to the vessel wall to ensure stable contact. Low-energy radiofrequency ablations are then applied moving the catheter from distal to proximal locations in the renal arteries.5
Anatomical and physiological effects of renal denervationThe renal nerves arise from T10-L2 and follow the course of the renal artery lying in the adventitia. Renal sympathetic nerves play an important role in the development of hypertension, mediated via sodium and water retention, increased renin release, and alterations in renal blood flow.5
Experimental studies established the important concept that sub-vasoconstrictor levels of renal sympathetic activity can increase renin secretion and renal sodium retention without changing renal hemodynamics.6 Assessment of regional overflow of norepinephrine (NE) from the kidneys to plasma has demonstrated that renal NE spillover rates can be markedly elevated in patients with essential hypertension and are associated with hypertensive end-organ damage.5,31 Measurement of NE excretion in the urine, now largely obsolete, was performed in order to quantify sympathetic nervous system activity in humans.6 In the light of these findings, RDN therapy was considered a logical therapeutic approach in the treatment of hypertension.
The sympathetic nervous system and cardiovascular disease7Surgical sympathectomy was first introduced in the 1930s and was effective in lowering high BP in patients with severe hypertension.8,9 However, the side effects associated with the procedure and the introduction of ganglionic blockers made it obsolete.10 For a long time, it was difficult to study and assess the role of the sympathetic nervous system (SNS) in the pathogenesis of hypertension, due not to uncertainty concerning the relation between the two, but rather to the difficulty of testing and assessing that relation. Previous techniques included the measurement of serum and urinary excretion of NE and its derivatives, yielding a rough estimate of SNS activity in the whole body.11 Hagbarth and Vallbo reported microneurography as a newer tool to study SNS nerve firing in subcutaneous tissues and skeletal muscles.12 The NE spillover technique was first used by Esler et al.,13 measuring organ-specific NE release in its efferent veins as an indirect measurement of SNS nerve fiber firing in an individual organ,14 and that technique was later used to demonstrate that heart failure caused SNS overactivity.15 Accumulated evidence underlines the role of sustained chronic SNS activity in many diseases including ischemic heart disease,16 heart failure,17,18 hypertension,19–21 kidney disease,22 type 2 diabetes,23 obesity,23 metabolic syndrome,23 obstructive sleep apnea,24 depression,25 and inflammatory bowel disease.26
In the cardiovascular system, chronic SNS activation not only raises BP but also causes hypertrophy and proliferation of vascular smooth muscle cells and cardiac myocytes, thus increasing LV mass and wall thickness.27 In addition, increased SNS activity, in the presence of endothelial dysfunction and endothelial cell damage, has been shown to contribute to the development of atherosclerosis.25,27,29 Contradicting previous assumptions, high levels of NE spillover from the heart in heart failure28 demonstrated increased sympathetic activity in the failing heart, which was the rationale for the subsequent use of beta-blockers in heart failure.29 In essential hypertension, SNS outflow to the kidneys is elevated and is directly proportional to the severity of hypertension.19,21,25 This finding is also supported by regional measurements of NE spillover to the kidneys.30 Renal sympathetic activity is a major factor contributing to the pathogenesis of essential hypertension through its influence on activation of the renin-angiotensin system, sodium and water excretion, peripheral vasoconstriction, cardiac contraction, and venous capacitance.25 The recent introduction of safe and efficient methods for RDN has led to a renewed interest in the role of SNS activation in essential hypertension.
Evidence of the efficacy of renal denervation in the treatment of resistant hypertensionThe Rapid Renal Sympathetic Denervation for Resistant Hypertension Using the OneShot™ Ablation System (RAPID) study32 was a prospective, multicenter, non-randomized, single-arm trial that included 50 patients with mean office BP of 181±20.8/95±15.5 mmHg, on a mean of five antihypertensive medications, who received RDN. The primary endpoint studied was the rate of office systolic BP (SBP) reduction: ≥10 mmHg at six months in comparison to baseline. Change in 24-hour ambulatory BP (ABP) was also evaluated. At the six-month mark, there was a decrease in mean office BP of -20/-8 mmHg (p<0.0001/p=0.0002) in comparison to baseline. This trend was sustained at 12 months, with a decrease in mean office BP of -22/-8 mmHg (p<0.0001/p=0.0014). Likewise, there was a significant reduction in 24-hour ABP, of -11/-6 mmHg at six months in comparison to baseline (p=0.0085/p=0.037). At 12 months, the reduction in 24-hour ABP was -9/-5 mmHg (p=0.054/p=0.073). Device- and/or procedure-related adverse effects occurred in three patients up to 12 months, namely one access site infection, one renal artery stenosis, and one groin paresthesia. The authors concluded that RDN led to significant and sustained reductions in office and 24-hour ambulatory BP at six and 12 months, respectively.
The REDUCE-HTN study33 was a prospective, non-randomized, single-arm trial that included 146 patients with mean baseline office and ambulatory BP of 182±18.4/100±14.0 and 153±15.1/87±13.2 mmHg, respectively, who received RDN using the Vessix renal denervation system. The primary efficacy endpoints were reductions in office and 24-hour ambulatory SBP and diastolic blood pressure (DBP) at six months, which were 24.7±22.1/10.3±12.7 mmHg (p<0.0001) and 8.4±14.4/5.9±9.1 mmHg (p<0.0001), respectively. Regarding the safety endpoints, no acute renal injury requiring acute renal intervention occurred and only one patient developed renal artery stenosis that required a stent. In addition, mean glomerular filtration rate remained stable. The authors concluded that renal artery denervation reduced both office and ambulatory BP at six months in patients with resistant hypertension.
The EnligHTN I study34 was a prospective, non-randomized trial that included 46 patients with mean baseline office, 24-hour ambulatory and home BP of 176/96, 150/83 and 158/90 mmHg, respectively, who underwent RDN using the EnligHTN multielectrode radiofrequency ablation system. The primary efficacy endpoint was change in office BP in comparison to baseline. The mean reductions in office BP at 1, 3, 6, and 12 months were -28/-10, -27/-10, -26/-10, and -27/-11 mmHg, respectively (p<0.001 for all). Likewise, mean reductions in 24-hour ABP were -10/-5, -10/-5, and -10/-6 mmHg at 1, 3, 6 months (p<0.001 for all), and -7/-4 mmHg at 12 months (p<0.0094). Mean reductions in home measurements were -9/-4, -8/-5, -10/-7, and -11/-6 mmHg (p<0.001 at 12 months). There were no signs of worsening renal function at 12 months. One patient required renal artery stenting (non-occlusive renal stenosis was present at baseline). The authors concluded that the EnligHTN ablation system was safe and effective in reducing office, 24-ambulatory and home BP in patients with resistant hypertension.
Oslo RDN35 was a prospective randomized trial in which 19 patients with true treatment-resistant hypertension were randomized to RDN (n=9) performed with the Symplicity Catheter System vs. clinically adjusted drug treatment (n=10). True treatment-resistant hypertension was confirmed after excluding patients with confounding poor drug adherence. The primary endpoint was change in office systolic blood pressure (SBP) from randomization to six months. The study was stopped early as RDN had uncertain BP-lowering effects. At six months, office SBP and DBP changed significantly from 160±14/88±13 mmHg to 132±10/77±8 mmHg compared to baseline in the drug-adjusted group (p<0.0005 and p=0.02), whereas in the RDN group there was no significant change in office SBP and DBP at six months compared to baseline (156±13/91±15 to 148±7/89±8 mmHg, p=0.42 and p=0.48). SBP and DBP were significantly lower in the drug-adjusted group at six months (p=0.002 and p=0.004, respectively), and absolute changes in SBP were larger in the drug-adjusted group (p=0.008). ABP changed in parallel to office BP. The authors concluded that the BP-lowering effect of adjusted drug treatment were superior to that of RDN in patients with true treatment-resistant hypertension.
The Prague-15 study36 was a prospective, open-label multicenter trial in which 106 patients with resistant hypertension were randomized to RDN (n=52) or intensified pharmacological treatment (n=54). RDN was performed using the Symplicity Renal Denervation System. At six months, a comparable significant reduction in mean 24-hour SBP was noted in both groups, of -8.6 mmHg (95% confidence interval [CI]: -11.8, -5.3); p<0.001 in RDN, vs. -8.1 (95% CI: -12.7, -3.4) mmHg in the pharmacological group. Likewise, a significant reduction in office SBP was observed in both groups mmHg (-12.4 [95% CI: -17.0, -7.8]; p<0.001 in RDN vs. -14.3 mmHg [95% CI: -19.7, -8.9]; p<0.001 in the pharmacological group). The authors concluded that RDN was safe; however in the setting of true resistant hypertension with confirmed compliance, it was not proved superior to intensified pharmacological treatment.
The DENERHTN trial37 was a prospective, open-label randomized controlled trial that assigned 106 patients to receive either RDN plus a standardized stepped-care antihypertensive treatment (SSAHT) regimen (RDN group, n=53) or SSAHT alone (control group, n=53). Prior to randomization, treatment resistance was confirmed by ABP monitoring after patients received oral antihypertensives for a period of four weeks. The primary endpoint studied was the mean change in daytime SBP from baseline to six months as assessed by ambulatory BP monitoring. The safety outcomes were the incidence of acute adverse events of the RDN procedure and the change in estimated glomerular filtration rate from baseline to six months. At six months, the mean change in daytime ambulatory SBP was -15.8 mmHg (95% CI -19.7 to -11.9) in the RDN group and -9.9 mmHg (-13.6 to -6.2) in the group receiving SSAHT alone, a baseline-adjusted difference of -5.9 mmHg (-11.3 to -0.5; p=0.0329). The numbers of antihypertensive drugs and drug adherence at six months were similar between the two groups. Three minor RDN-related adverse events were noted (lumbar pain in two patients and mild groin hematoma in one patient). The authors concluded that RDN plus SSAHT decreases ambulatory BP more than the same SSAHT alone at six months in patients with well-defined resistant hypertension.
The Symplicity HTN studiesThe Symplicity HTN-1 study38 was a proof-of-principle trial of therapeutic renal sympathetic denervation. The trial included 50 patients with resistant hypertension who received percutaneous radiofrequency catheter-based RDN using the Symplicity catheter, with a one-year follow-up post procedure. The primary endpoints were office BP and safety data before and at 1, 3, 6, 9, and 12 months after procedure. Out of the 50 patients enrolled, five were excluded for anatomical reasons. Treated patients were on a mean of 4.7 antihypertensive medications, with baseline mean office BP of 177/101 mmHg. Office BP readings after the procedure were reduced by -14/-10, -21/-10, -22/-11, -24/-11, and -27/-17 mmHg at 1, 3, 6, 9, and 12 months, respectively. In the five non-treated patients, mean rise in office BP was +3/-2, +2/+3, +14/+9, and +26/+17 mmHg at 1, 3, 6, and 9 months, respectively. The only reported complication was one intraprocedural renal artery dissection which occurred before radiofrequency energy delivery. The authors concluded that catheter-based RDN leads to substantial and sustained BP reduction, without serious adverse events, in patients with resistant hypertension.
Following the success achieved in the Symplicity HTN-1 trial, the Symplicity HTN-2 trial39 was designed to assess the effectiveness and safety of catheter-based RDN for BP reduction in patients with treatment-resistant hypertension. A total of 106 patients with resistant hypertension were randomly assigned to either RDN with medical treatment (treatment arm, n=52) or medical treatment alone (control arm, n=54). The primary endpoint of the study was change in office-based measurement of SBP at six months. At six months, there was a significant reduction in office-based BP measurements in the treatment arm (change of 32/12 mmHg, standard deviation [SD] 23/11, baseline of 178/96 mmHg), compared to the control arm (change of 1/0 mmHg, SD 21/10, baseline of 178/97 mmHg). Between-group differences in BP at six months were 33/11 mmHg (p<0.0001). Additionally, at six months, 41 (84%) out of 49 patients in the treatment arm had a reduction in SBP of 10 mmHg or more, compared with 18 (35%) of 51 controls (p<0.0001). No serious procedural complications were noted. The authors concluded that catheter-based RDN can safely be used to substantially reduce BP in treatment-resistant hypertensive patients. Follow-up at 36 months in 40 of the 52 patients in the initial RDN group and at 30 months in 30 of 37 patients who crossed over and received RDN at six months40 showed that SBP decreased by 34 mmHg (95% CI: -40, -27, p<0.01) and DBP decreased by 13 mmHg (95% CI: -16, -10, p<0.01) from a baseline of 184±19/99±16 mmHg. SBP and DBP reductions at 36 months for the initial RDN group was -33 mmHg (95% CI: -40, -25, p<0.01) and -14 mmHg (95% CI: -17, -10, p<0.01), respectively.
In an attempt to further explore the robustness of RDN in the treatment of resistant hypertension, the Symplicity HTN-3 trial41 was conducted. Unlike the previous two studies, blinding and sham-control were added in the design of this trial. A total of 535 patients with severe resistant hypertension receiving a stable antihypertensive regimen involving maximally tolerated doses of at least three drugs including a diuretic were randomly assigned in a 2:1 ratio to undergo RDN (n=364) or a sham procedure (n=171).
The primary efficacy endpoint, change in office SBP at six months, was not significantly different between the two groups (-14.13±23.93 mmHg in the denervation group compared to -11.74±25.94 mmHg in the sham-procedure group [p<0.001 for both comparisons of the change from baseline], a difference of -2.39 mmHg [95% CI: -6.89 to 2.12; p=0.26 for superiority with a margin of 5 mm Hg]). Likewise, for the secondary efficacy endpoint, the change in mean 24-hour ambulatory SBP, no between-group differences were noted -6.75±15.11 mmHg in the denervation group and -4.79±17.25 mmHg in the sham-procedure group, for a difference of -1.96 mmHg (95% CI: -4.97 to 1.06; p=0.98 for superiority with a margin of 2 mmHg). The primary safety endpoint (a composite of death, end-stage renal disease, an embolic event resulting in end-organ damage, renovascular complications, or hypertensive crisis within 30 days or new renal-artery stenosis of more than 70% within six months) was not significantly different between the two groups. The authors concluded that their blinded trial did not show a significant reduction of SBP in patients with resistant hypertension six months after renal-artery denervation compared with a sham control.
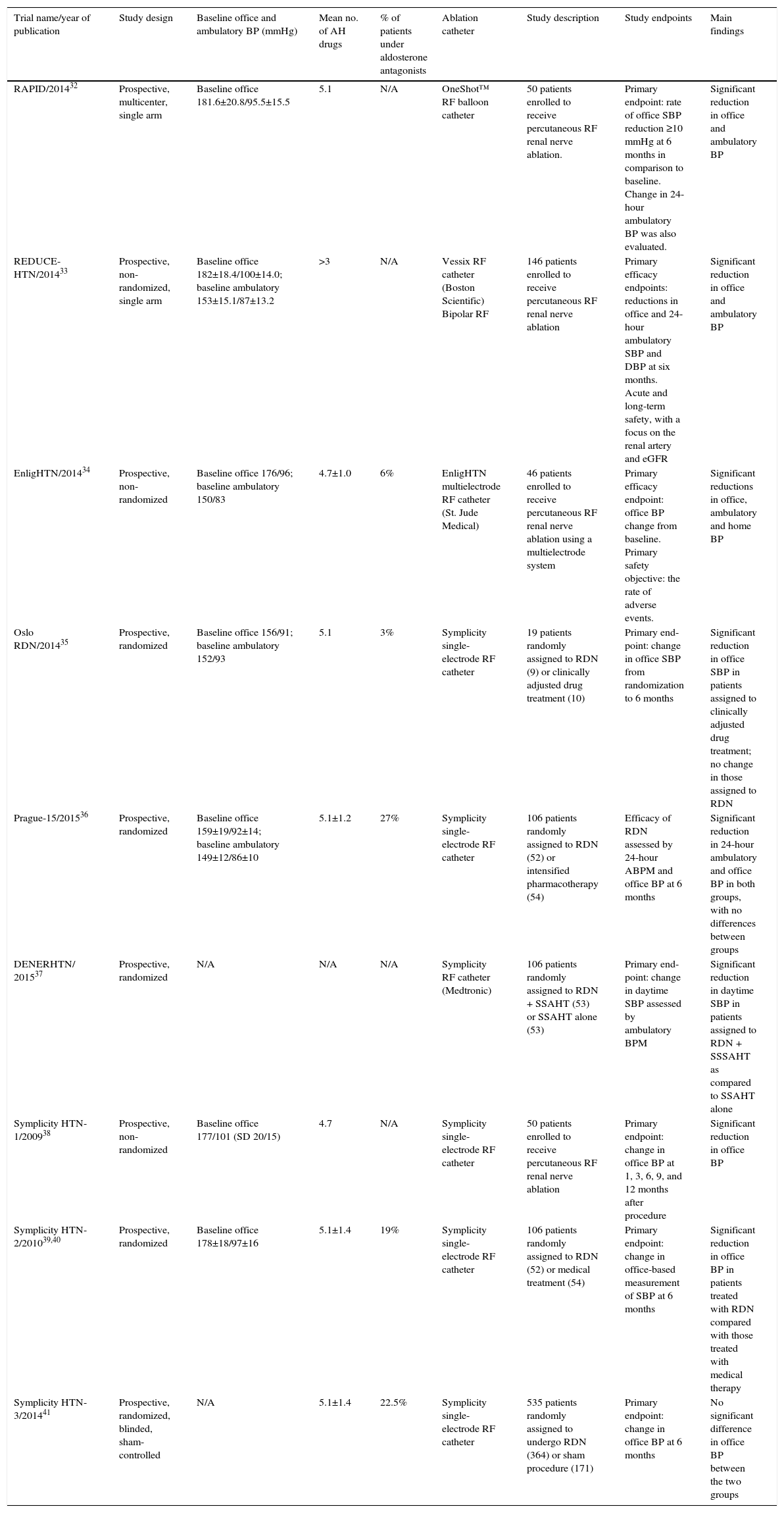
Table 1 shows a summary of trials of RDN.
Summary of trials of renal denervation.
| Trial name/year of publication | Study design | Baseline office and ambulatory BP (mmHg) | Mean no. of AH drugs | % of patients under aldosterone antagonists | Ablation catheter | Study description | Study endpoints | Main findings |
|---|---|---|---|---|---|---|---|---|
| RAPID/201432 | Prospective, multicenter, single arm | Baseline office 181.6±20.8/95.5±15.5 | 5.1 | N/A | OneShot™ RF balloon catheter | 50 patients enrolled to receive percutaneous RF renal nerve ablation. | Primary endpoint: rate of office SBP reduction ≥10 mmHg at 6 months in comparison to baseline. Change in 24-hour ambulatory BP was also evaluated. | Significant reduction in office and ambulatory BP |
| REDUCE-HTN/201433 | Prospective, non-randomized, single arm | Baseline office 182±18.4/100±14.0; baseline ambulatory 153±15.1/87±13.2 | >3 | N/A | Vessix RF catheter (Boston Scientific) Bipolar RF | 146 patients enrolled to receive percutaneous RF renal nerve ablation | Primary efficacy endpoints: reductions in office and 24-hour ambulatory SBP and DBP at six months. Acute and long-term safety, with a focus on the renal artery and eGFR | Significant reduction in office and ambulatory BP |
| EnligHTN/201434 | Prospective, non-randomized | Baseline office 176/96; baseline ambulatory 150/83 | 4.7±1.0 | 6% | EnligHTN multielectrode RF catheter (St. Jude Medical) | 46 patients enrolled to receive percutaneous RF renal nerve ablation using a multielectrode system | Primary efficacy endpoint: office BP change from baseline. Primary safety objective: the rate of adverse events. | Significant reductions in office, ambulatory and home BP |
| Oslo RDN/201435 | Prospective, randomized | Baseline office 156/91; baseline ambulatory 152/93 | 5.1 | 3% | Symplicity single-electrode RF catheter | 19 patients randomly assigned to RDN (9) or clinically adjusted drug treatment (10) | Primary end-point: change in office SBP from randomization to 6 months | Significant reduction in office SBP in patients assigned to clinically adjusted drug treatment; no change in those assigned to RDN |
| Prague-15/201536 | Prospective, randomized | Baseline office 159±19/92±14; baseline ambulatory 149±12/86±10 | 5.1±1.2 | 27% | Symplicity single-electrode RF catheter | 106 patients randomly assigned to RDN (52) or intensified pharmacotherapy (54) | Efficacy of RDN assessed by 24-hour ABPM and office BP at 6 months | Significant reduction in 24-hour ambulatory and office BP in both groups, with no differences between groups |
| DENERHTN/ 201537 | Prospective, randomized | N/A | N/A | N/A | Symplicity RF catheter (Medtronic) | 106 patients randomly assigned to RDN + SSAHT (53) or SSAHT alone (53) | Primary end-point: change in daytime SBP assessed by ambulatory BPM | Significant reduction in daytime SBP in patients assigned to RDN + SSSAHT as compared to SSAHT alone |
| Symplicity HTN-1/200938 | Prospective, non-randomized | Baseline office 177/101 (SD 20/15) | 4.7 | N/A | Symplicity single-electrode RF catheter | 50 patients enrolled to receive percutaneous RF renal nerve ablation | Primary endpoint: change in office BP at 1, 3, 6, 9, and 12 months after procedure | Significant reduction in office BP |
| Symplicity HTN-2/201039,40 | Prospective, randomized | Baseline office 178±18/97±16 | 5.1±1.4 | 19% | Symplicity single-electrode RF catheter | 106 patients randomly assigned to RDN (52) or medical treatment (54) | Primary endpoint: change in office-based measurement of SBP at 6 months | Significant reduction in office BP in patients treated with RDN compared with those treated with medical therapy |
| Symplicity HTN-3/201441 | Prospective, randomized, blinded, sham-controlled | N/A | 5.1±1.4 | 22.5% | Symplicity single-electrode RF catheter | 535 patients randomly assigned to undergo RDN (364) or sham procedure (171) | Primary endpoint: change in office BP at 6 months | No significant difference in office BP between the two groups |
ABPM: ambulatory blood pressure monitoring; AH: antihypertensive; BP: blood pressure; eGFR: estimated glomerular filtration rate; N/A: not applicable; RDN: renal denervation; RF: radiofrequency; SSAHT: standardized stepped-care antihypertensive treatment; SD: standard deviation.
The role of RDN in the treatment of resistant hypertension is still to be fully clarified. While the results of the RAPID, REDUCE-HTN, EnligHTN, Symplicity HTN-1 and -2, and DENERHTN trials were positive, this was not the case in the Oslo RDN, Prague-15 and Symplicity HTN-3 trials. The varying results may be explained by several factors. Symplicity HTN-3, the largest trial to date, was also the only one that included a sham-control group. As shown by Meissner et al.,46 sham procedures may be associated with a significant positive response – the placebo effect – that could be misinterpreted as a real treatment effect. Kandzari et al.42 investigated potential reasons for the discordant results noted in Symplicity HTN-3. Their findings showed that between randomization and the six-month endpoint, 39% of patients underwent medication changes involving alteration in both the dose and class of prescribed medications. In addition, African-Americans were more likely to be prescribed vasodilators than non-African-Americans in both study arms. A total of 56% of African-American sham patients and 46.7% of African-American RDN patients were receiving a vasodilator, compared to 40.5% of non-African-American sham control patients and 33.7% of non-African-American RDN patients who were receiving a vasodilator at baseline. African-Americans in the sham control study arm receiving a vasodilator showed an enormous decline in SBP (-21.9+29.1 mmHg), which was not noted in the other subgroups. This unexpectedly large SBP decline in African-American control patients may indicate a change in medical adherence or type of therapy in this specific group. Likewise, increasing number of ablations and energy delivery in a four-quadrant pattern was associated with greater reductions in office and ambulatory SBP and heart rate.
The ablation catheters used in the above studies had some design features that are worthy of mention. The EnligHTN denervation system is a unique multielectrode catheter that allows a predictable pattern of four simultaneous ablations. The Vessix denervation system used in the REDUCE-HTN trial delivered bipolar radiofrequency energy as opposed to the monopolar radiofrequency energy delivered by the Symplicity denervation system (used in the Symplicity HTN studies). As suggested by Kandazari et al.,42 differences in ablation energy may affect the efficacy of denervation therapy.
The futureSeveral trials are underway to investigate the role of RDN in specific patient populations such as those with chronic renal disease and moderate hypertension.43–45
The SPYRAL HTN Global Clinical Trial Program47 is a phased series of clinical trials designed to establish the efficacy of RDN therapy in patients with hypertension. The SPYRAL HTN-OFF MED trial aims to confirm the basic hypothesis that RDN therapy lowers BP in patients with hypertension without treatment, while the parallel SPYRAL HTN-ON MED trial is designed to assess the efficacy of RDN integrated with antihypertensive medications.
ConclusionsThe results of the RAPID, REDUCE-HTN, EnligHTN, Symplicity HTN-1 and -2, and DENERHTN trials suggested an important role for RDN in the treatment of resistant hypertension. The results of the Oslo RDN, Prague-15 and Symplicity HTN-3 suggested differently. Of note, all these studies showing favorable benefits of RDN were either non-randomized single-arm trials or randomized unblinded trials without a sham control group. As shown by Meissner et al.,46 sham procedures may be associated with a significant positive response – the placebo effect – that could be misinterpreted as a real treatment effect. Symplicity HTN-3, a well-designed randomized, blinded, sham control trial, casts doubt on the benefits of RDN in the treatment of resistant hypertension. The results of the Symplicity HTN-3 emphasize the need always to assess for a potential placebo effect by including sham control study groups when assessing the efficacy of new devices. In addition, future studies should ensure consistent medical therapy and delivery of radiofrequency ablation across treatment arms, as post-hoc analysis showed these differences to be important in the neutral results noted in Symplicity HTN-3.
Currently, the role of RDN in addition to medical therapy in the treatment of resistant hypertension is still to be fully clarified. Nevertheless, RDN is certainly not dead, but further well-designed clinical trials are needed to ascertain the benefits or otherwise of RDN in treatment-resistant hypertension.
Conflicts of interestThe authors have no conflicts of interest to declare.