We report a case of sarcoidosis with an unusual presentation, initially manifesting as bilateral pulmonary embolism and then as a cardiac form of the disease with an ominous clinical event consisting of sustained ventricular tachycardia. The diagnosis was established by clinical and magnetic resonance criteria despite normal conventional echocardiographic study. Detailed functional assessment provided by tracking techniques (speckle tracking echocardiography and cardiac magnetic resonance tissue tracking) enabled the detection of regional deformation abnormalities, indicating prominent circumferential strain and epicardial layer alterations, partly matching the structural changes depicted by distribution of delayed enhancement.
We find this case notable for various issues it raises concerning diagnosis and management of cardiac sarcoidosis. These are mainly related to recent developments in imaging modalities that enable non-invasive identification of structural and functional abnormalities in this condition early, before overt deterioration in left ventricular ejection fraction. Information from different imaging modalities and tools provide information that could potentially assist preclinical diagnosis, with possible prognostic implications.
Apresenta-se o caso de um doente de 44 anos com o diagnóstico prévio de sarcoidose pulmonar, admitido consecutivamente no serviço de urgência por embolia pulmonar e taquicardia ventricular sintomática. Embora o estudo ecocardiográfico mostrasse normal fração de ejeção ventricular esquerda, o diagnóstico de sarcoidose cardíaca foi estabelecido por elementos de ordem clínica, em conjugação com os achados de ressonância magnética cardíaca. Quando se efetuou estudo funcional detalhado do ventrículo esquerdo por speckle (ecocardiografia) e tissue tracking (ressonância magnética), detetaram-se alterações regionais da deformação miocárdica, parcialmente coincidentes com a distribuição do realce tardio por ressonância. Para além da apresentação pouco habitual sob a forma de evento embólico, este caso de sarcoidose com subsequente documentação de envolvimento cardíaco, permitiu a aplicação de modalidades de imagem cardiovascular avançadas, colocou em evidência a afeção estrutural e funcional miocárdica, na presença de normal fração de ejeção ventricular esquerda.
Sarcoidosis is a multisystem inflammatory disease of unknown etiology, with heterogeneous presentation and possible cardiovascular involvement. Associated mortality is mainly due to respiratory, neurological or cardiac complications. Myocardial involvement, which may manifest as heart block, ventricular arrhythmias or heart failure from both systolic and diastolic dysfunction, may have life-threatening consequences, and cardiac sudden death may be the first presentation of cardiac sarcoidosis, occurring independently of pulmonary or other organ involvement. When sarcoidosis is fatal, cardiac involvement is a frequent cause of death1,2. Recently, an association between sarcoidosis and pulmonary embolism has been described, which is explained by inflammatory and other biochemical mechanisms3,4. This could worsen the prognosis of both pulmonary and cardiovascular disease and makes it important to seek early identification of patients at risk.
It has become clear that asymptomatic cardiac involvement is far more prevalent that previously thought. Nevertheless, there is a lack of consensus as to the diagnostic and cardiovascular imaging modalities to be used and their relative accuracy in identifying the presence of preclinical cardiac disease.
Conventional two-dimensional (2D) echocardiography is recommended for assessing sarcoidosis patients with suspicion of cardiac involvement. However, morphological changes and overt global ventricular dysfunction, as assessed by left ventricular ejection fraction (LVEF), probably occur simultaneously with already established clinical cardiac manifestations.
Speckle tracking echocardiography (STE) is a valuable tool for the quantitative assessment of regional myocardial function. As in its early stages cardiac sarcoidosis does not affect the myocardium uniformly or globally, the disease could theoretically be identified by this technique, possibly before overt deterioration in LVEF2. In keeping with the mainly regional nature of the disease, cardiac magnetic resonance (CMR) is currently one of the advanced high-resolution imaging techniques of choice in the assessment of sarcoidosis, enabling rapid, accurate, and non-invasive diagnosis. Some studies have set out to find correlations between functional myocardial changes as assessed by STE and scar distribution on contrast-enhanced (CE) CMR5. Furthermore, as tissue regions are identified by individual anatomical features in CMR, feature- and tissue-tracking CMR has been explored in 2D cine image stacks, and a series of deformation parameters describing myocardium mechanics can also be derived6.
We present a case report of cardiac sarcoidosis with a rare presentation, preceded by extensive pulmonary embolism. Although the diagnosis was made by CE-CMR, we proceeded with further segmental functional analysis by STE, investigating possible effects on regional function. We also sought to assess myocardial deformation by tissue-tracking CMR and the extent of its agreement with STE.
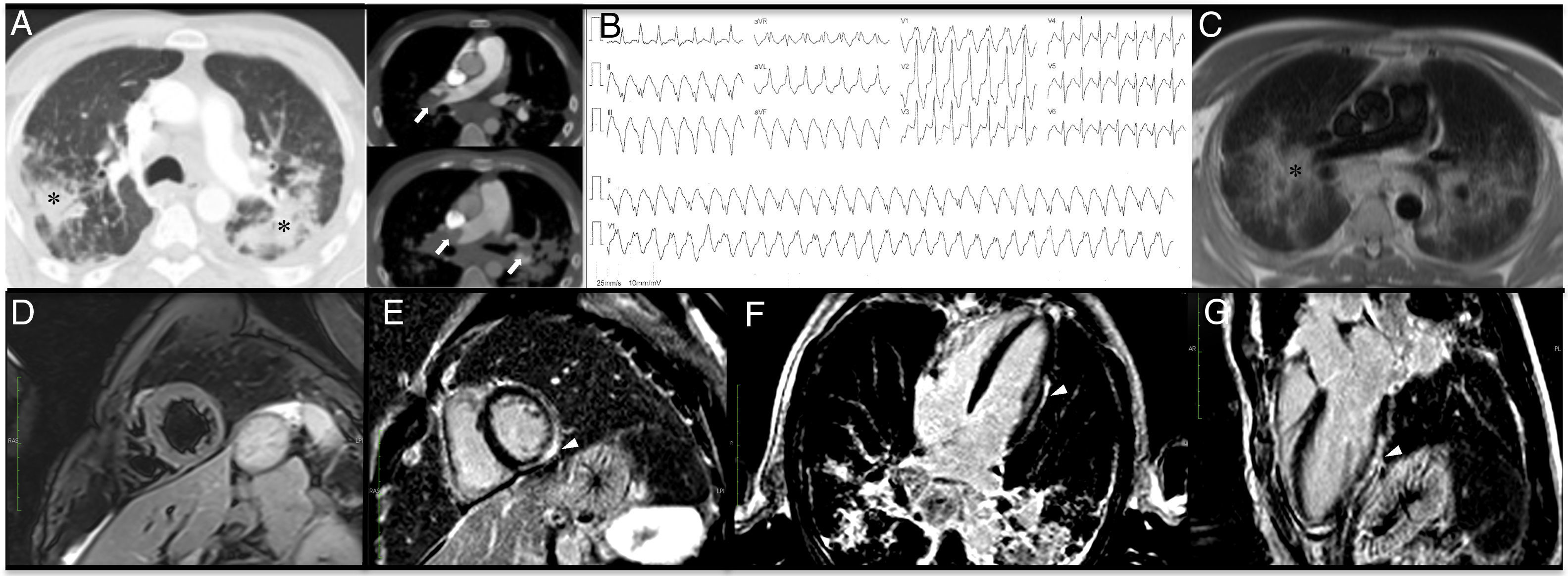
Case reportA 44-year-old black male with pulmonary sarcoidosis diagnosed at the age of 39, under irregular corticosteroid therapy, presented to the hospital with sudden onset of effort dyspnea. On admission the patient was in no distress, with tachycardia (100 beats/min) and normal blood pressure and oxygen saturation (ambient air). Physical examination was negative except for the presence of bilateral inspiratory crackles in the middle third of both lung fields. The electrocardiogram (ECG) revealed no changes other than sinus tachycardia, and blood analysis was remarkable for D-dimer elevation with normal BNP levels. The patient underwent pulmonary computed tomography (CT) angiography, which revealed bilateral pulmonary embolism in addition to parenchymal nodular infiltration, interstitial ground glass pattern and bilateral mediastinal lymphadenopathy with several lymph node conglomerates (Figure 1A). Transthoracic echocardiography was normal, with no signs of acute right-sided pressure overload or of right ventricular dysfunction. Lower limb Doppler ultrasound was positive for partial right popliteal vein thrombosis. The patient was started on oral anticoagulation with rivaroxaban in addition to corticotherapy; hospital stay was uneventful and he was discharged.
(A) Thoracic computed tomography: pulmonary window with evidence of severe and diffuse parenchymal nodular infiltration (asterisk), also depicted in (C), and bilateral pulmonary artery thrombosis, almost obstructive at the level of the right pulmonary artery (white arrow); (B) monomorphic ventricular tachycardia notable for a maximum deflection index of >55% in the precordium (and an intrinsicoid deflection time of >85 ms), indicating epicardial origin. Because there is right bundle branch block morphology in V1 and QS in leads II, III and aVF, the origin is in the inferior/inferolateral left ventricle; (C) cardiac magnetic resonance axial localizer; (D) T2-weighted turbo-spin echo, short-axis sequence, negative for the presence of edema; (E-G) phase-sensitive inversion recovery sequences depicting delayed enhancement sparing the subendocardium across the inferior, lateral and inferolateral wall of the left ventricle (arrowhead).
Six weeks after admission he returned to the emergency department complaining of sudden dizziness. He was hypotensive and tachycardic, with no other significant findings. His ECG was notable for monomorphic ventricular tachycardia (Figure 1B), which was successfully cardioverted to sinus rhythm. Blood tests were negative for both D-dimers and troponin. Notwithstanding, pulmonary CT was repeated, which showed partial resolution of pulmonary thrombosis, with similar parenchymal and lymph node changes. Conventional transthoracic echocardiography revealed normal LVEF. Owing to suspicion of cardiac sarcoidosis, CE-CMR was requested. It showed no myocardial edema but was positive for delayed enhancement sparing the subendocardial layers, and confirmed normal biventricular volumes and LVEF (Figure 1C-G). The patient accordingly received an implantable cardioverter-defibrillator, and was asymptomatic at six-month follow-up with no further ventricular tachycardia episodes, under oral anticoagulation. A detailed coagulation investigation conducted subsequently, including screening for antiphospholipid antibodies, was negative.
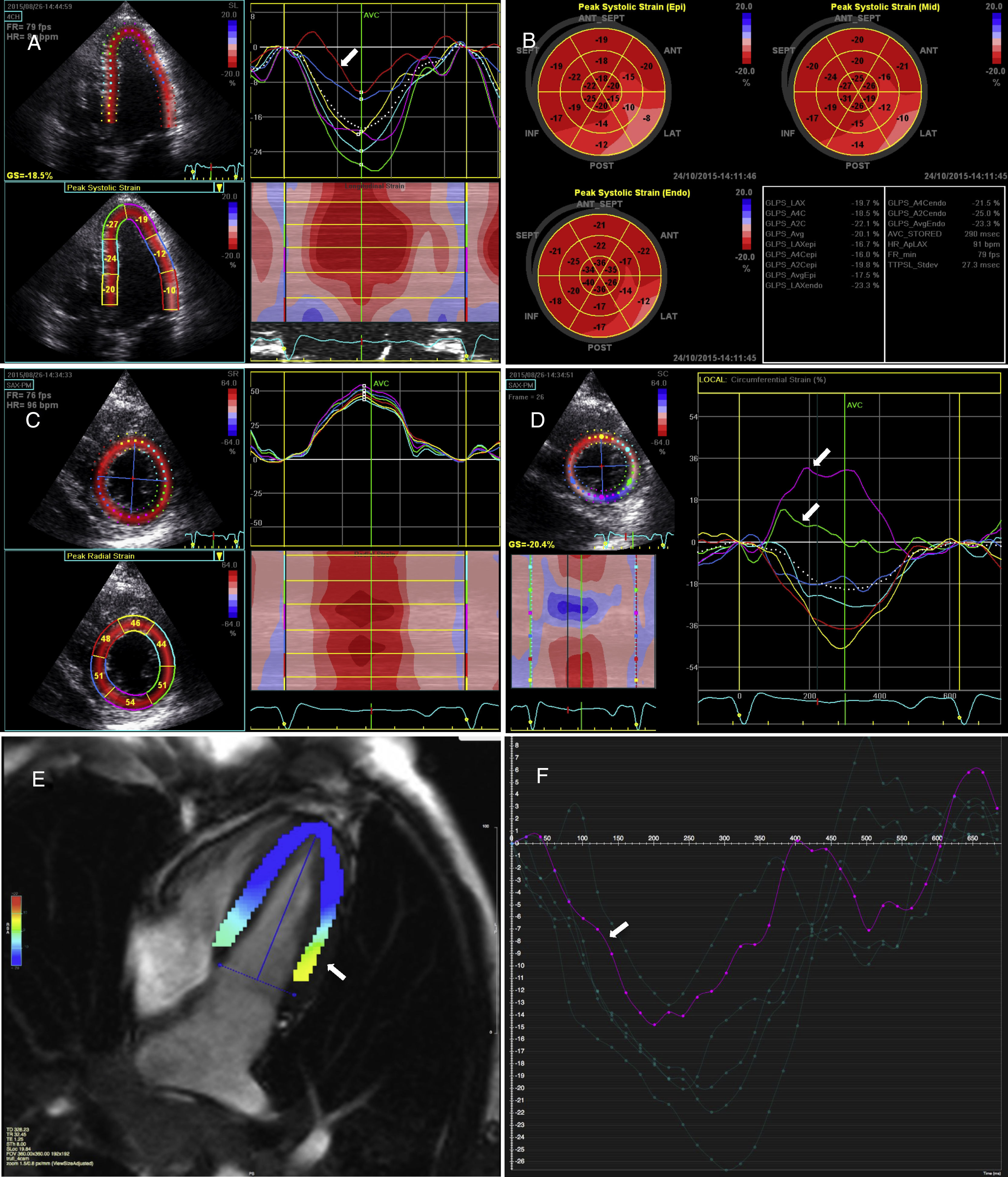
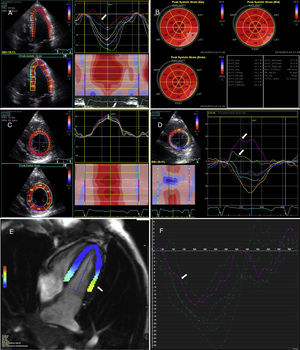
After the CMR study a detailed echocardiographic analysis was performed. Global longitudinal strain was assessed as normal despite slightly lower regional values at the basal and mid lateral (Figure 2A) and inferior-lateral walls, and this abnormality was increasingly noticed when detailed mid and epicardial longitudinal strain were analyzed (Figure 2B), with slightly abnormal global longitudinal strain at the latter layer. In accordance with these findings, and even more pronounced than the longitudinal strain, we found abnormal circumferential strain values at the same segments, with positive values for the mid left ventricular subepicardial layers, fully matching the distribution of delayed enhancement (Figure 2C and Figure 3). Nevertheless, radial strain values were normal at the same level and segments, not indicating regional deformation abnormalities.
(A) Longitudinal strain assessed in 4-chamber view, with evidence of reduced strain at the basal and mid lateral wall (arrow); (B) global longitudinal strain at endocardial (Endo), mid-myocardium (Mid) and epicardial (Epi) levels; abnormal values towards the lateral and inferior lateral walls are increasingly noticed from endocardial to epicardial views, with slightly below normal global longitudinal epicardial strain (-17.5%); (C) radial strain curves and values at the level of the papillary muscles; (D) epicardial circumferential strain curves at the level of the left ventricular papillary muscles with markedly abnormal values (positive strain) at the inferior and inferolateral wall (arrows); (E and F) tissue tracking cardiac magnetic resonance (cvi42 version 5 software, Circle Cardiovascular Imaging Inc., Calgary, Canada) showing abnormal deformation at the basal lateral wall (yellow-green map, arrow) (E) with the corresponding strain curve (arrow) (F).
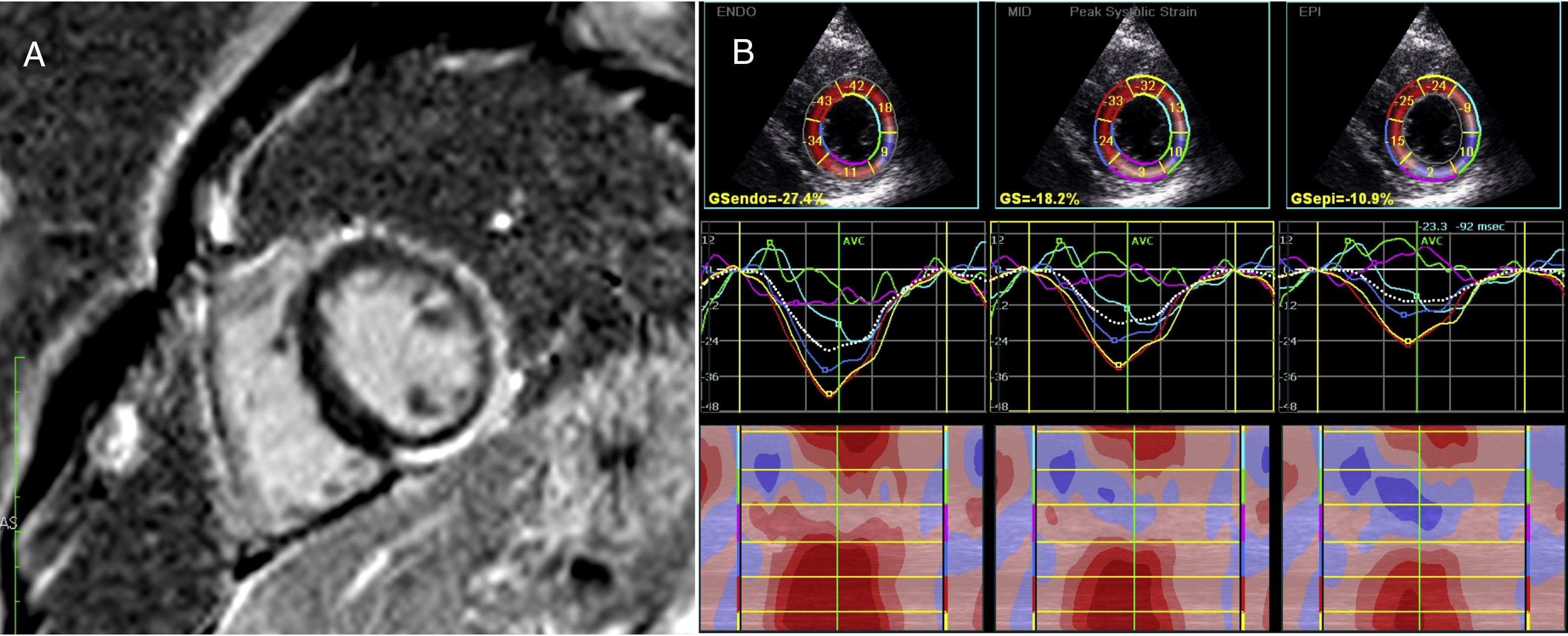
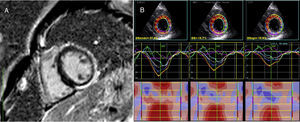
(A) Phase-sensitive inversion recovery sequence (left ventricular short axis at the level of the papillary muscles) showing overt non-ischemic delayed enhancement matching abnormal circumferential strain findings at the same level (B), especially noticed when detailed epicardium (EPI) layer analysis is performed, as provided by speckle tracking echocardiography. ENDO: endocardial; GS: global (circumferential) strain; MID: mid-myocardial.
At tissue tracking CMR, regional longitudinal deformation also matched STE longitudinal strain alterations at the basal lateral wall (Figure 2E and F), although radial and circumferential strain were unchanged (Videos 1 and 2). However, on global four-dimensional tissue tracking, radial strain assessment was notable for slight alterations in basal lateral wall strain (Video 3).
DiscussionSarcoidosis is a chronic granulomatous disease mainly affecting patients between the ages of 20 and 40 years. Cardiac involvement has been demonstrated in 20-50% of patients in autopsy studies but clinically manifest cardiac disease is observed in only about 5% of those with cardiac involvement. As in the case presented, black individuals are more likely than other racial groups to suffer extrathoracic organ involvement and a chronic disease course1. It has also recently been demonstrated that sarcoidosis may predispose to venous embolic events and its association with pulmonary embolism is significant regardless of age, gender or race. In this case pulmonary embolism was the first clinical event raising suspicion of sarcoidosis involvement beyond merely the lung parenchyma and mediastinum. The cause of this potential increase in risk of pulmonary embolism is the subject of speculation, but could include effects of corticosteroid treatment, the unrecognized presence of disease-specific procoagulant factors (increased thrombin activation and fibrin formation derived from macrophages and activated leukocytes), simultaneous presence of antiphospholipid antibodies (found in up to 38% of sarcoidosis patients), which was not documented in this report, and even local vein compression by lymphadenopathy, resulting in blood stasis4.
This patient also presented with one of the most feared cardiac complications of the disease. Symptomatic ventricular tachycardia was the episode that led to the diagnosis of cardiac sarcoidosis despite the previous normal findings at conventional 2D echocardiography. Asymptomatic left ventricular dysfunction, proving regional sarcoidosis involvement, was only established following advanced imaging studies. Although negative for edema, CMR displayed delayed enhancement with a non-ischemic pattern, which not only supported the diagnosis but also provided further evidence for scarring and substrate heterogeneity predisposing to ventricular arrhythmias. Even so, this delayed enhancement pattern was not typical, as it is characteristically patchy, with involvement of the basal septum, in this context2.
Global longitudinal strain assessed by STE as a measure of left ventricular function was within normal values. However STE was remarkable for heterogeneity of regional dysfunction, largely of longitudinal strain measured at the mid myocardial and epicardial layers, but particularly of circumferential strain, epicardial values of which were clearly abnormal at the inferior and inferolateral left ventricular wall. Notwithstanding, radial strain analysis was within normal values at the same left ventricular level, which did not identify regional dysfunction following this strain assessment.
By definition, STE is influenced by myocardial heterogeneity in laminar extension, shear and thickening. Coronary artery disease studies have demonstrated that the spatial sensitivity of high-resolution STE enables focused assessment of specific layers of the myocardium and a graded assessment of parts of the myocardial wall with different sensitivity to reduced coronary perfusion. Although the relation between flow and transmural strain in three dimensions (longitudinal, circumferential and radial) is non-linear, different sensitivities can be attributed to a single strain dimension in order to assess a specific myocardial layer5. In agreement with this, segmental strain analysis to investigate myocarditis revealed the predominantly subepicardial and intramyocardial involvement in this case, mainly of oblique and circumferentially oriented fibers, which would explain the circumferential strain abnormalities. Both longitudinal and circumferential strain reduction in the acute phase have proved to be of prognostic value for the occurrence of events and subsequent recovery of LVEF5. Significant changes in both longitudinal and circumferential strain are thus to be expected in this case of sarcoidosis, with a typical non-ischemic myocardial pattern of involvement, although with no evidence of edema as in acute myocarditis. However, in contrast to our findings, radial strain would need integrity of cross-fiber activation of all layers and should therefore have shown significant changes. Moreover, a single study with three-dimensional STE showed that only radial strain assessment had good potential to distinguish cardiac sarcoidosis from dilated cardiomyopathy, which should increase interest in strain analysis in this condition6. This finding may bear some relation to previous contradictory data from assessment of possible overlap between areas of reduced strain and delayed late gadolinium enhancement. Although we found obvious matching of distribution of changes in circumferential strain and delayed enhancement, this has not generally been the case in previous reports on cardiac sarcoidosis, and usually there are more areas of reduced strain than areas with enhancement. As STE and tracking techniques reflect quantitative mechanics, it is possible that subclinical cardiac involvement has subtle effects on myocardial contractility early, before structural lesions such as granulomas or fibrosis occur with sufficient spatial resolution to be detected by available tissue characterization sequences that have limited sensitivity. In addition, if this was the case, specific technical features of tracking algorithms, such as temporal averaging and stronger endocardial weighing for CMR tissue tracking, may explain the different findings for all other strain dimensions as assessed by echocardiography and CMR5,6. Although the issue is complex, we should point out that possible correlation of strain analysis with delayed enhancement distribution is merely speculative in this case in particular and for cardiac involvement in sarcoidosis in general. Data concerning these issues are scarce and the main findings are derived from studies on coronary artery disease and myocarditis.
One final point should be made concerning imaging data and prognosis. Both left ventricular dilatation as assessed by echocardiography and myocardial delayed enhancement are independent predictors for overall mortality and risk of sudden cardiac death and arrhythmic events, respectively. In one study, alterations in global longitudinal strain were the best independent echocardiographic predictor of worse outcome in cardiac sarcoidosis patients2. However, it should be noted that there are no specific data addressing cardiac structural and functional involvement in patients at different stages of the disease. While identification of cardiac involvement before overt deterioration in LVEF could be improved, it is still unknown whether regional alterations and delayed enhancement occur before left ventricular dilatation and heart failure. Even so, in this case, ventricular arrhythmias occurred in the presence of normal volumes and LVEF.
Overall, this case report is notable for the unusual clinical presentation and the value of the different available imaging tools for diagnosis of cardiac sarcoidosis. Our experience shows that reliance should not be placed exclusively on LVEF in this setting. STE, being less resource-intensive, less costly, portable and easily performed, should be performed to detect alterations in regional strain that would suggest the diagnosis. Moreover, CMR can provide a definitive structural diagnosis if its findings correlate with strain values. As a technique with many applications, CMR with appropriate and experienced use of feature-tracking techniques may also provide functional data for regional strain analysis in the future.
Broadly speaking, more work is needed using different applications and models for both functional and strain analysis and tissue characterization with different imaging modalities before they can be routinely used on a daily basis. The aim should be a systematic analysis of how all these tools are to be applied, not merely for optimizing the diagnosis of cardiac sarcoidosis but, more importantly, for detecting preclinical cardiac alterations before the occurrence of serious clinical events.
Conflicts of interestThe authors have no conflicts of interest to declare.