Quadricuspid aortic valve (QAV) is a rare congenital condition that frequently progresses to aortic regurgitation with clinical impact in adulthood. Surgical treatment is required in the fifth to sixth decade of life in about one fifth of patients.
We describe the case of a 64-year-old woman with regular cardiological follow-up for severe aortic valve regurgitation who had suffered recent clinical and echocardiographic deterioration. Conventional open surgery was indicated. During the procedure, a QAV with leaflet retraction and central orifice was observed. The aortic valve was successfully replaced.
A válvula aórtica quadricúspide é uma malformação congênita rara. A progressão para insuficiência aórtica com significado clínico é frequente na idade adulta. O tratamento cirúrgico, quando indicado, tem habitualmente lugar por volta da quinta ou sexta décadas de vida em cerca de um quinto dos doentes.
Descrevemos o caso clínico de uma doente de 64 anos com diagnóstico de regurgitação valvular aórtica severa e deterioração clínica e ecocardiográfica, reunindo critérios para cirurgia convencional. Durante a cirurgia, observou-se uma válvula aórtica composta por quatro folhetos independentes, retraídos e com má coaptação central. O procedimento decorreu sem intercorrências.
We report the case of a 64-year-old woman with regular cardiological follow-up for severe aortic valve regurgitation. She complained of fatigue and dyspnea with moderate exertion, dizziness and sporadic palpitations, with recent clinical (New York Heart Association functional class II-III) and echocardiographic deterioration. She had a previous history of hypertension, dyslipidemia, overweight, asthma and Sjögren syndrome, and was on diuretics, but with no previous hospitalizations for heart failure.
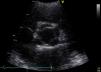
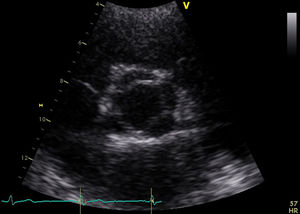
She was in sinus rhythm (∼80 bpm) with a diastolic murmur at the apex. The chest X-ray was normal with preserved cardiothoracic index. The preoperative echocardiogram revealed slightly enlarged left chambers (left atrium 46 mm; left ventricular systolic/diastolic diameters 41/59 mm; interventricular septal systolic/diastolic dimensions 11/15 mm, respectively) and preserved contractility (ejection fraction 63%). The aortic valve had four leaflets with preserved opening (no transvalvular gradient was present) but poor coaptation causing severe aortic regurgitation (vena contracta 8 mm) (Figure 1 and Video 1). The ascending aorta measured 36 mm.
Cardiac catheterization revealed a slightly dilated ascending aorta (42 mm) and an incompetent aortic valve causing severe aortic regurgitation. No coronary or carotid disease was found.
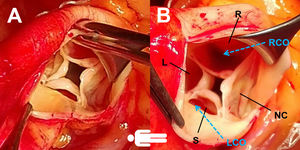
The patient was operated electively. In the operating room, a concentrically hypertrophied left ventricle, dilated ascending aorta and fibrosed quadricuspid aortic valve (QAV) with leaflet retraction and a central orifice were observed (Figure 2). The supernumerary leaflet was the smallest and the others were of equal size. The left coronary ostium was tunneled under the commissure, which warranted special care in order to avoid damage during excision of the valve or obstruction by the prosthesis. Cardioplegia was delivered antegradely, directly in the coronary ostia. We also routinely use topical ice slush or cold saline solution as an adjuvant to myocardial protection. A 21-mm St. Jude mechanical prosthesis was implanted and the surgery ended uneventfully. The predischarge echocardiogram showed preserved ejection fraction (50%) and the mechanical aortic valve with normal opening and no paravalvular leak. Transvalvular gradients (maximum/mean) were 22/12 mmHg. No other valve lesions or significant pericardial effusion were found. The patient was discharged on the fifth postoperative day.
Surgical photographs (A and B) clearly showing a four-cusp aortic valve, with poor central coaptation and two independent ostia. The supernumerary leaflet was the smallest and the others were of equal size (type B and III according to Hurwitz and Roberts’3 and Nakamura et al.’s6 classifications, respectively). The left coronary ostium was close to and tunneled under the commissure. L: left coronary cusp; LCO: left coronary ostium; NC: non-coronary cusp; R: right coronary cusp; RCO: right coronary ostium; S: supernumerary cusp.
Quadricuspid semilunar valves are rare, especially QAV, which is found in about 0.008% in autopsy series, 0.043% in echocardiogram findings and incidentally in 0.05-1% of patients undergoing surgery due to aortic regurgitation. In our experience, this is the second case in the last decade, in which we performed more than 4000 aortic valve procedures (0.0005%). This aortic valve morphology is less frequent than bicuspid (2%) and unicuspid valves.1 Quadricuspid pulmonary valves are also uncommon, but usually function well.4
QAV is often dysfunctional, with clinical impact in adulthood, which suggests that the initial congenital size or shape anomalies are not severe, but degenerate and become symptomatic later. Thus, surgical treatment is usually required in the fifth to sixth decade of life in about one fifth of patients.1–3 Tsang et al. described a group of 50 patients with echocardiographic diagnosis of QAV during a five-year follow-up period, during which only eight of them needed surgery.2
There is a slight male predominance (1.6:1). Pure aortic regurgitation is predominant (75%), due to fibrous thickening and incomplete coaptation. Mixed aortic valve disease occurs in 9% of cases and 16% are functionally normal.1
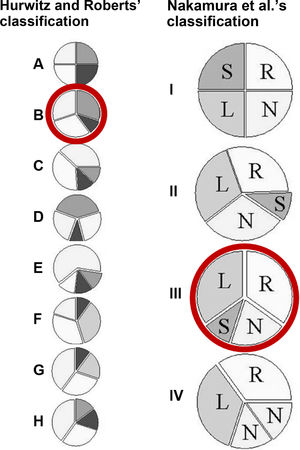
Hurwitz and Roberts classified seven types (A-G) of QAV according to the relative size of the cusps.3 Later, Vali et al.4 added an eighth type (H) (Figure 3). Types A, B and C comprise 85% of cases. Nakamura et al.6 described a simpler classification using the supernumerary cusp position (I-IV) (Figure 3). No association between QAV morphology and severity of aortic regurgitation has been established.7 According to Hurwitz and Roberts’3 and Nakamura et al.’s6 classifications, our patient was a type B (three equal-sized and one smaller cusp) and type III, respectively. The smallest (and supernumerary) cusp was between the left and non-coronary cusps.
Left: the seven (A-G) anatomic types of quadricuspid valves described by Hurwitz and Roberts. Type H was added later by Vali et al.5. The most common is type B and the rarest is type D. Right: the simplified classification of Nakamura et al., based on the position of the supernumerary cusp. L: left coronary cusp; N: non-coronary cusp; R: right coronary cusp; S: supernumerary cusp. Red circles identify the case presented in this report.
The embryological origin of QAV is uncertain, but the mechanisms involve aberrant septation of the conotruncus and leaflets.3 Ostia and coronary artery anomalies are often present. The left ostium is most frequently displaced and surgeons should take this into account.8 In our case, the left coronary ostium was close to and tunneled under the commissure, so that there was a risk of damage to it during valve excision and obstruction by the valve sewing cuff (Figure 2).
Table 1 presents an overview of surgical QAV cases reported in the literature, with ostia displacement or coronary abnormalities.
Overview of reported surgical quadricuspid aortic valve cases with coronary abnormalities.
| Study | n | Age | Gender | Type of QAVa | Indication for surgery | Ostia or coronary abnormalities | Type of surgery and technical implications |
|---|---|---|---|---|---|---|---|
| Holm et al.11 | 1 | 44 y | F | A | Severe AR | LCA ostium unusually low in the aortic root near the posterior margin of the left coronary cusp | Replacement with a low-profile mechanical prosthesis in order to avoid interference with the low ostium |
| Mutsuga et al.12 | 1 | 10 y | F | C | MI due to occlusion of LCA ostium | Emergency surgery showed a QAV with a small left-side cusp partially adhering to the aortic wall, blocking blood flow to the LCA | Resection of the small cusp adhering to the wallThe discrepancy in the size of the cusps and MI forced aortic valve replacement |
| Wang et al.13 | 1 | 53 y | M | A | Severe AR | Ostia of LAD and RCA were juxtaposed at the right coronary sinus LAD coursed between the aortic root and the RVOT. The LCx originated from the proximal segment of the RCA, coursing posterior to the aortic root, and then into the atrioventricular groove | NA |
| Okamoto et al.14 | 1 | 68 y | F | B | Giant coronary artery aneurysm; mild AR | Giant coronary artery aneurysm and pulmonary artery fistulas extending from the LCA and RCA | Resection of the aneurysm and aortocoronary bypass with LIMA; the valve was not approached |
| Hayakawa et al.15 | 1 | 70 y | M | B | Severe AR | Origin of the RCA near the commissure between left and right coronary cusps | Replacement by bioprosthesis |
| Idrees et al.9 | 3 | NA | NA | NA | Severe aortic valve disease (and root dilatation) | Partial occlusion of the ostium by the commissure (n=2); LCA arising from the noncoronary sinus (n=1) | Partial occlusion corrected by valve repair; displaced ostium was implanted as a button in the native position during root replacement |
| Tsang et al.4 | 1 | 16 y | NA | NA | Occlusion of the left coronary ostium; mild to moderate AR | Occlusion of the LCA ostium by a small fourth cusp and collateralization by the RCA | Excision of the obstructive cusp and suspension of the others; reperfusion by the LCA was documented postoperatively |
| Gupta et al.8 | 1 | 64 y | F | A | Severe AR | Two LCA ostia due to early bifurcation of LCA; both were located very close to the commissures | Replacement by bioprosthesis |
| Kim et al.16 | 1 | 56 y | F | A | Severe AR | Single oval ostium due to both coronary arteries arising in the left coronary sinus | Replacement by mechanical prosthesis |
| Harada et al.17 | 1 | 4 m | M | NA | Poor cardiac function (LVEF <20%); mild AR | LCA ostium located below commissure between two noncoronary cusps, creating an ostial obstruction as a membrane with two tiny holes; poor right collateral vessels | Native LCA ostium below commissure was closed and translocated above, using a pericardial patch |
| Das et al.18 | 1 | 27 y | M | B | Severe AR | RCA ostium located near the accessory fourth cusp | Replacement by mechanical prosthesis |
According to Hurwitz and Roberts’ classification.3
AR: aortic regurgitation; AS: aortic stenosis; LAD: left anterior descending coronary artery; LCA: left coronary artery; LCx: left circumflex coronary artery; LIMA: left internal mammary artery; LVEF: left ventricular ejection fraction; m: months; MI: myocardial infarction; NA: not available; QAV: quadricuspid aortic valve; RCA: right coronary artery; RVOT: right ventricular outflow tract; y: years.
Other cardiac disorders that may be found together with QAV include enlargement of the ascending aorta, septal defects, patent ductus arteriosus, pulmonary stenosis, fenestrations of the sinus of Valsalva, tetralogy of Fallot, nonobstructive hypertrophic cardiomyopathy, subaortic stenosis, transposition of the great arteries and persistent left superior vena cava.2,7
Idrees et al.9 described their surgical experience with 31 QAV patients over a 21-year period. They showed that pure aortic regurgitation is predominant and repair is feasible in some cases with good outcomes (only one reoperation was needed). Repair techniques include resection of the dysfunctional/accessory leaflet or plication and commissural closure (tricuspidization) and bicuspidization (commisuroplasty of two pairs of adjacent cusps, when there are two small cusps). A Ross procedure is also an option. However, most undergo aortic valve replacement.10 There are few reports of quadricuspid valve repair and even fewer reporting long-term outcomes concerning the durability of repair in this context. It was for this reason that aortic valve replacement was performed in the present case.
Infective endocarditis is found in 1.4% of cases. It is not clear whether QAV increases the risk of infection in the same way as is observed in bicuspid valves.1 It is thought that when the valve has four equal cusps, the risk of endocarditis is low due to the symmetry, reducing flow disturbance.
Conflicts of interestThe authors have no conflicts of interest to declare.