Surgical treatment for chronic thromboembolic pulmonary hypertension (CTEPH) is challenging. Most Portuguese patients with CTEPH have been referred to foreign institutions for treatment, with significant social and economic costs. To meet this emerging need, the cardiothoracic surgery department of Hospital de Santa Marta, Lisbon, has developed a dedicated program for pulmonary thromboendarterectomy (PTE). We hereby present the results for the first 19 patients treated.
MethodsWe conducted a retrospective analysis of all 19 patients who underwent PTE at Hospital de Santa Marta between 2008 and April 2019.
ResultsSince 2008, a total of 19 patients have undergone PTE in our department. The procedure was performed with good outcomes in both survival and functional recovery. At the very beginning of the series two patients died perioperatively, before all the team underwent formal training at the Royal Papworth Hospital, UK, with no early deaths since. Postoperative complications were similar to other published series. During 11 years of follow-up, there were three late deaths, all in patients with residual pulmonary arterial hypertension. At the latest follow-up (October 2019), all surviving patients showed significant functional recovery, all in NYHA class I or II, with only one patient on vasodilator therapy with sildenafil (the first in the series, operated in 2008).
ConclusionsPTE is a demanding procedure, in which outcomes are related to volume and accumulated experience, however it can be performed safely and with reproducible results by a properly prepared dedicated team with a well-controlled learning curve. More patients and multidisciplinary experience will be needed to further improve and streamline results.
O tratamento cirúrgico da hipertensão pulmonar tromboembólica crónica (CTEPH) é desafiante. Até ao presente, a maioria dos doentes portugueses sofrendo desta patologia têm vindo a ser referidos para tratamento em instituições estrangeiras, com importantes custos sociais e económicos. Para responder à necessidade de desenvolvimento de um tratamento local de qualidade para estes doentes, o Serviço de Cirurgia Cardiotorácica do Hospital de Santa Marta (HSM) tem vindo a desenvolver um programa dedicado de tromboendarterectomia pulmonar (PTE). Apresentamos os resultados com os primeiros 19 doentes.
MétodosAnálise retrospetiva de todos os 19 doentes submetidos a PTE no HSM de 2008 a abril de 2019.
ResultadosDesde 2008, 19 doentes foram tratados no Serviço de Cirurgia Cardiotorácica do HSM, com bons resultados, quer a nível da sobrevivência quer da recuperação funcional. As complicações pós-operatórias são semelhantes às descritas na literatura. Ocorreram duas mortes perioperatórias no início da experiência, antes de a equipa ter feito um período de treino formal no Royal Papworth Hospital, sem mortalidade precoce desde então. Durante os 11 anos de follow-up, ocorreram três mortes tardias, em doentes com algum grau de hipertensão pulmonar residual. À data do último seguimento (outubro de 2019), os doentes vivos apresentavam recuperação funcional significativa, encontrando-se todos em classe NYHA I ou II. Apenas um (o primeiro desta série, operado em 2008) estava sob terapêutica vasodilatadora pulmonar com sildenafil.
ConclusõesA PTE é um procedimento exigente, em que os resultados estão dependentes de volume de casos e acumulação de experiência, mas que pode ser realizada com segurança e resultados reprodutíveis por uma equipa dedicada com uma curva de aprendizagem bem controlada. Mais doentes e experiência multidisciplinar serão necessários para melhorar e otimizar os resultados.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a form of pulmonary arterial hypertension (PAH) resulting from the fibrotic transformation of pulmonary artery clots causing chronic obstruction of the pulmonary arteries, leading to vascular remodeling in the microvasculature. Consequently, pulmonary arterial pressure and vascular resistance increase, leading inexorably to right heart failure, functional impairment and premature death.
Not all patients with CTEPH report a history of acute pulmonary embolism (PE); the incidence of CTEPH after PE is around 1.5% and registry data indicate a prevalence of 3-30 per million in the general population.1 However, most CTEPH patients present with a previous PE. The International CTEPH Registry reports a previous acute PE event in 74.8% of CTEPH patients, and the cumulative incidence is reported to be 0.1-9.1% within two years of any symptomatic pulmonary embolic event.
Although the exact prevalence and annual incidence of CTEPH are unknown, some data suggest that the condition may occur in approximately five per million population per year. Its prevalence in European countries is 3.2 cases per million per year, and is 0.9 cases per million in Spain,2 which, due to similarities in the populations of the two countries, means that a reasonable estimate for Portugal would be nine new cases yearly.
The true incidence and prevalence of CTEPH in the Portuguese population are unknown, although according to Gouveia et al.3 the estimated incidence of acute pulmonary embolism in Portugal in 2013 was 35 per 100 000 population. In a nationwide study by Baptista et al.,4 after excluding patients in World Health Organization (WHO) PAH groups 2, 3 and 5, CTEPH patients comprised 41.8% of all those with PAH. This study, based on a small number of patients followed at dedicated PAH centers, remains the sole nationwide data available for CTEPH.
Predisposing factors such as thrombophilic disorders, lupus anticoagulant and antiphospholipid antibody syndromes, protein S and C deficiency, activated protein C resistance including factor V Leiden mutation, thrombotic gene mutations, antithrombin III deficiency and elevated factor VIII have been reported in 31.9% of patients and previous splenectomy in 3.4%.2
Diagnosis and assessment of operabilityA diagnosis of CTEPH is based on findings obtained after at least three months of effective anticoagulation, in order to differentiate this condition from subacute forms of PE.2
The clinical criteria for diagnosis according to the 2015 European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines were mean pulmonary artery pressure (mPAP) ≥25 mmHg (revised in 2018 to ≥20 mmHg), pulmonary artery wedge pressure ≤15 mmHg, and specific diagnostic signs for CTEPH on imaging studies. These include mismatched perfusion defects on lung scan (a normal V/Q scan effectively excludes CTEPH with a sensitivity of 90-100% and specificity of 94-100%), and ring-like stenoses, webs/slits and chronic total occlusions (pouch lesions or tapered lesions).2 Imaging studies – multidetector computed tomography (CT) angiography, magnetic resonance imaging (MRI), or conventional pulmonary cineangiography – are essential for diagnosis and lesion characterization and to guide surgical intervention. At least two imaging methods are recommended; elective pulmonary cineangiography can be replaced by MRI or CT scan.
In addition to imaging studies, patients should undergo right heart catheterization, an echocardiogram, and cardiorespiratory functional testing such as the 6-min walk test or treadmill exercise testing.6,7
Acceptance criteriaPulmonary thromboendarterectomy (PTE) is the treatment of choice for patients with CTEPH, both for symptomatic relief and for improvement or normalization of pulmonary hemodynamics.
There is general agreement in the literature that PTE should be offered to every operable patient with CTEPH if the risk is acceptable, since this provides survival benefit.
According to the guidelines, operability is determined by multiple factors that are not easily standardized. Certain features such as the degree of pulmonary vascular resistance (PVR) and location of thrombotic lesions are strongly related to outcomes.8,9
To be considered operable, a patient must have sufficient surgically accessible thromboembolic material, and more importantly, a good correlation between the extent of disease and the degree of PVR. Extensive secondary peripheral vasculopathy should be excluded.1,6,9
Patients of advanced age, frail or in poor general condition, or with serious comorbidities, are generally deemed to be inoperable due to their overall surgical risk. Operability is best established based on a case-by-case analysis, and borderline cases will certainly need a second opinion.
Although registry data suggest that in the past more than one third of patients diagnosed with CTEPH did not proceed to PTE surgery, PTE should be offered to the majority of patients.1,6,9,10
Although PTE is the treatment of choice for CTEPH, it is still not widely available to the Portuguese population, with only a small number of patients being referred to international centers, at considerable personal and societal cost.11
Since 2008, the cardiothoracic surgery department of Hospital de Santa Marta in Lisbon has been operating a PTE program, providing surgical treatment to Portuguese patients with CTEPH. The department provides the full range of services needed to perform and support this type of surgery, including cardiothoracic surgery, structural cardiology, cardiac and thoracic anesthesiology, extracorporeal membrane oxygenation (ECMO) and lung transplantation programs. We hereby present the initial experience with this ongoing program.
MethodsWe retrospectively analyzed data from all patients who underwent PTE at our center. These patients were referred by our local PAH team, as well as by other expert PAH teams in the country.
All patients were diagnosed and assessed according to the ESC/ERS guidelines, at least two imaging tests (CT scan and pulmonary angiography) being required to determine the diagnosis and establish operability.
Baseline patient characteristics are described in Table 1.
Clinical characteristics of the study population at diagnosis (n=19).
| Demographics | |
| Age, years | 54.8±14.8 |
| Male gender | 36.8 (7) |
| BMI, kg/m2 | 26.8±5.1 |
| Genetics (confirmed) | 21 (4) |
| Preoperative | |
| Creatinine, mg/dl | 0.76±0.23 |
| Systemic hypertension | 68.42 (13) |
| Diabetes | 15.8 (3) |
| Smoking history | 26.3 (5) |
| COPD | 15.8 (3) |
| Previous MI | 5 (1) |
| Previous acute event | 31.6 (7) |
| Clinical | |
| NYHA I/II | 5 (1) |
| NYHA≥III | 94.7 (18) |
| Use of pulmonary vasodilators | 21 (4) |
| Supplementary O2 | 36.8 (7) |
| Echocardiography | |
| PASP, mmHg | 100.5±14.84 |
| TAPSE, mm | 14.5±0.7 |
| TR grade>III | 47.4 (9) |
| Hemodynamics | |
| mPAP, mmHg | 45.6±13.4 |
| PASP, mmHg | 81.4±24.3 |
| PVR, WU | 22.8±6.8 |
| PVR, dyn.s.cm5 | 1821.6±575.1 |
| PVR>12.5 WU/1000 dyn.s.cm5 | 42 (8) |
| CI, l/min/m2 | 2.02±0.68 |
Values are percentage (n) or mean±standard deviation.
One patient had a previous myocardial infarction treated with percutaneous coronary intervention of the left anterior descending artery.
BMI: body mass index; CI: cardiac index; COPD: chronic obstructive pulmonary disease; MI: myocardial infarction; mPAP: mean pulmonary artery pressure; NYHA: New York Heart Association class; PASP: pulmonary artery systolic pressure; PVR: pulmonary vascular resistance; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid regurgitation; WU: Wood units.
All patients were operated electively, by the same surgical team, following the same standard perioperative protocol.
All procedures were performed with aortic and double venous cannulation, right superior pulmonary vein and main pulmonary artery venting, single-shot cold blood cardioplegia and standard circulatory arrest periods of 20 min at 18°C nasopharyngeal temperature. If circulatory arrest periods longer than 20 min were needed, 10-min reperfusion periods were mandatory between arrest periods.
Standard modified ultrafiltration and cerebral protection measures were routinely used.
The central pulmonary arteries were mobilized up to the hilum, taking care not to open the pleurae, then, under periods of complete hypothermic circulatory arrest and total exsanguination, pulmonary endarterectomies were carried out sequentially, first on the right, then on the left side.
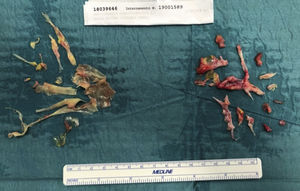
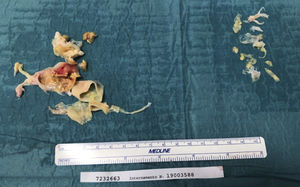
The critical step is the development of the correct dissection plane, followed by meticulous circumferential dissection, extending distally to the segmental and subsegmental branches. This enables the removal of full casts and webs, leading to effective relief of obstruction (Figures 1-3).
After direct closure of the pulmonary arteriotomies with 5-0 nylon sutures, patients are fully rewarmed and weaned off cardiopulmonary bypass. Inotropic support is used according to our protocol, typically in low to moderate dosages. Meticulous hemostasis is achieved.
Only the first patient (in 2008) underwent tricuspid annuloplasty; none of the other patients had their functional tricuspid regurgitation addressed at surgery, since recovery is expected after removing right ventricular afterload, as stated in the literature.12
Operative specimens are systematically documented (Figures 1-3).
Mean surgical times are displayed in Table 2 and some operative specimens are shown in Figures 1 and 2.
Postoperative managementPostoperative care is conducted according to published protocols, paying particular attention to prevention and management of reperfusion edema.
Inotropic support use was determined by hemodynamic and echocardiographic monitoring. Low-dose dobutamine and noradrenaline are most frequently used, other inotropes or vasodilators being added according to clinical circumstances.
Negative fluid balance (by fluid restriction and intravenous furosemide) is crucial in the management of these patients, as is early adequate anticoagulation. Anticoagulant therapy consists of low molecular weight heparin (1 mg/kg, twice daily), started as early as possible (ideally in the first 24 hours if surgical bleeding is <50 cc/h), and switched over to oral anticoagulation with vitamin K antagonists, until therapeutic international normalized ratio levels (around 3) are achieved.
Postoperative data are summarized in Table 3.
Postoperative data.
| Hospital stay | |
| ICU stay, days | 9.2±8.5 |
| Total stay, days | 21.5±12.9 |
| Complications | |
| Pulmonary steal | 31.6 (n=6) |
| Reperfusion edema | 5 (n=1) |
| ARDS | 5 (n=1) |
| Renal dysfunction | 5.26 (n=1) |
| Inotropes>24 h | 52 (n=10) |
| Bleeding>10 mg/kg/24 h and/or transfusion | 57.9 (n=10) |
| Discharge | |
| Home | 73.68 (n=14) |
| Other hospital | 26.31 (n=5; three patients later discharged home, two died in referring hospital)a |
| Mortality | |
| In-hospital mortality (<30 days) | 10.5 (n=2)a |
| Late mortality | 15.78 (n=3)b |
The mean age of the patients was 54.8±14.8 years. They were predominantly female (63.2%), had a history of a previous acute event in 31.6% of cases, and in 21% of cases there were confirmed genetic procoagulant conditions.
As expected, most patients (91.7%, n=14) presented with significant clinical and functional impairment, in New York Heart Association (NYHA) class III or IV. Oxygen supplementation was needed in 36.8% and 21% were on chronic pulmonary vasodilator therapy.
Echocardiography showed severe pulmonary hypertension and right ventricular dysfunction in all patients, 47.4% of patients presenting with severe (grade III or IV) tricuspid regurgitation. Hemodynamic data confirmed the echocardiographic findings, with a significant proportion of patients (42%) presenting with severely elevated PVR (>12.5 Wood units/1000 dyn.s.cm5). Cardiac output was uniformly low.
Baseline patient characteristics are described in Table 1.
Operative dataGiven the nature of this surgical technique, surgical times tend to be long, particularly due to the cooling and rewarming phases. Cross-clamp and circulatory arrest times were within the recommended limits.
No patients needed operative ECMO support.
Operative data are summarized in Table 2 and some operative specimens are shown in Figures 1 and 2.
Postoperative course and complicationsPostoperative course was frequently prolonged, with a mean intensive care unit stay of 9.2±8.5 days and total length of stay of 21.5±12.9 days.
Pulmonary complications were the most common, occurring in eight patients. Of these, six (31.6%) presented with some form of pulmonary steal syndrome with persistent hypoxemia, requiring prolonged ventilation or supplementary oxygen postoperatively. One patient presented with frank pulmonary reperfusion edema and one patient had full-blown acute respiratory distress syndrome. Renal dysfunction occurred in one patient, without need for renal replacement therapy.
Central nervous system complications were rare and if present were mild, presenting as mood changes and/or agitation.
Need for inotropes for more than 48 h was frequent, albeit at low dosages.
Transfusion was frequent, due to a relatively liberal transfusion strategy (transfusion threshold ≤8 g/dl hemoglobin), but no patients needed reoperation for bleeding.
ECMO support was not needed postoperatively.
Most patients (73.68%, n=14) were discharged home, with a small number (26.31%, n=5) being transferred to the referring hospitals due to delayed recovery. Of these, three patients were later discharged home having made an adequate recovery and two died at the referring hospital.
Due to the small sample number, these delayed recoveries weighed heavily in the length of stay numbers.
Follow-upComplete follow-up was achieved in all patients.
As to long-term outcomes, by October 2019, 14 patients were alive and all had experienced significant functional recovery, being in NYHA class I or II, and there had been consistent improvement in hemodynamic parameters (confirmed in some patients by right heart recatheterization). Only one patient is on vasodilator therapy, with sildenafil (the first in the series, operated in 2008). Although on sildenafil, this patient is currently in NYHA class II. Present numbers are still too small for any meaningful survival analysis.
MortalityTwo patients died perioperatively, in 2011 and 2012, before all of the team underwent formal training in the experienced center at the Royal Papworth Hospital, UK. One patient died from intraoperative pulmonary artery rupture and the other from fulminant hemoptysis on the sixth postoperative day. These two early deaths were probably related to the fact that they were operated early in our learning curve.
At 11 years of follow-up, three late deaths had occurred, all patients having in common moderate residual pulmonary hypertension. One patient, with ischemic cardiomyopathy and left ventricular dysfunction (left ventricular ejection fraction 40%) died two months postoperatively, from documented H1N1 infection followed by sepsis and renal failure. The other two patients were considered high risk, with severe functional limitation and extensive disease. Despite relief of pulmonary arterial obstruction, one of these patients died three months postoperatively from respiratory infection and failure. The third patient, with pre-existing severe lung parenchymal disease, suffered rethrombosis of the right pulmonary artery despite appropriate anticoagulation, and died from pneumonia and respiratory failure on postoperative day 36 in the referral hospital.
As suggested in the literature, late deaths may occur due to unrelated causes in up to 49% of patients, particularly those with some residual pulmonary hypertension and elevated PVR (≥425 dyn.s.cm5). This cutoff has been shown to correlate with worse survival10 and is in line with the late mortality in our series. In Table 4 and Figure 3, we review our late mortality cases in detail.
Late mortality: detailed analysis.
| Patient | Age, years | Gender and medical history | Preoperative status | Surgery | Dis | Dis to | D | TOD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PE | O2 | Rioc | mPAP, mmHg | PASP, mmHg | PVR, dyn.s.cm5 | CI, l/min/m2 | NT-proBNP>1000 pg/ml | ||||||||
| 1 | 72 | MalePrevious CAD with PCI of the LAD>70% in-stent lesionLVEF 40% | Y | N | N | 35 | 60 | 571 | 2.2 | Y | PTE+LIMA to LAD | PO$24 | Home | H1N1 infection and septic shock, renal failure | PO70 |
| 2 | 76 | FemaleCOPD | N | N | N | 60 | 98 | 1281 | 1.89 | Y | PTE | PO10 | Referring hospital | Respiratory infection and failure | PO60 |
| 3 | 48 | FemaleCOPDActive smoker (120 pack/years)StrokeBipolar diseaseSevere RV dysfunction | Y | Y | Y | 55 | 89 | 2581 | 1.77 | Y | PTE | PO20 | Referring hospital | Respiratory failure and infection | PO36 |
CAD: coronary artery disease; CI: cardiac index; COPD: chronic obstructive pulmonary disease; D: cause of death; Dis: time of discharge; Dist to: discharged to; LAD: left anterior descending artery; LIMA: left internal mammary artery; LVEF: left ventricular ejection fraction; mPAP: mean pulmonary artery pressure; N: no; O2: supplementary oxygen; PASP: pulmonary artery systolic pressure; PCI: percutaneous coronary intervention; PE: pulmonary embolism; PO: postoperative day; PTE: pulmonary thromboendarterectomy; PVR: pulmonary vascular resistance; Rioc: preoperative riociguat; RV: right ventricular; PASP: pulmonary artery systolic pressure; TOD: time of death; Y: yes.
This paper presents data on the initial experience with PTE performed at a single center in Portugal.
Results achieved so far by our center show acceptable survival and complication rates, well within the expected range, given the level of our present experience.
The importance of a comprehensive multidisciplinary approach cannot be overestimated. Such an approach has undoubtedly helped to smooth the effects of the learning curve, keeping patients safe and within expected functional outcomes.
As for any complex surgical procedure, PTE requires high surgical volumes and accumulation of experience in order to achieve reproducible results and clinical excellence, as stated in the updated recommendations from the 2018 Cologne Consensus Conference.13 In-hospital fatalities correlate inversely with the number of cases performed yearly. Specialized centers (those performing over 50 PTE procedures/year) show case fatality rates of ≤3.5%. For centers performing between 11 and 50 PTE procedures/year, mortality averages 4.7%, and for centers that perform fewer than 11 PTE/year it averages 7.4%. Our center is presently in the latter group.
The recommended minimum number of PTE procedures to be performed annually is 20; an expert center should perform at least 20 PTE operations per year with less than 10% mortality.6 Also in line with these recommendations, an experienced surgeon is defined as one who performed over 20 PTEs in the year they were first assessed, and cumulatively over 40 procedures in the previous three years.6,10
For Portugal, a yearly case load of 20 PTEs could easily be achieved, allowing for reproducible results and making Hospital de Santa Marta an expert center.
We believe that by increasing referrals, we are now building up a solid experience with PTE. Nevertheless, we might still occasionally have to refer more complex and challenging cases for surgery abroad, particularly those with severe associated comorbidities or more severe peripheral disease, in order to provide patients with the best possible treatment.
The recognition of our center as a national reference center for adult PAH and PTE, following a nationwide open contest, would enable us, in close collaboration with every PAH unit in Portugal, to build up a robust national PTE center. Only by increasing the PTE case load and closely monitoring our results and auditing our data in a transparent way will this aim be achieved.
ConclusionsThis is a brief report of an initial series of PTE procedures, performed at a single center. The numbers are still small, but results are encouraging, reflecting a rapidly rising learning curve, without undue complications or mortality.
Complication rates are consistent with those published in the literature, including transient pulmonary dysfunction, which occurred in less than half of cases.
Long-term outcomes showed a consistent improvement in NYHA class and hemodynamic parameters, with all patients in NYHA class I or II.
Late mortality occurred in a few patients, due to a combination of pre-existing clinical severity and residual, though moderate, pulmonary hypertension.
Present numbers are still too small for any meaningful long-term survival analysis.
We conclude that, with a multidisciplinary team, it is possible to start a local program for PTE and to manage the learning curve, with reproducible results.
Undoubtedly, more patients will be needed to further improve results, but we feel our initial findings are encouraging.
AuthorshipJosé Fragata: Surgeon performing the cases, critical revision and final approval of the version to be submitted.
Helena Telles: acquisition, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content.
The authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all those involved in the care of these patients, from referral centers to our own team, and Dr. Jorge Pinheiro Santos for editing the manuscript.