Heart failure is associated with high rates of readmission and mortality, and there is a need for measures to improve outcomes. This study aims to assess the impact of the implementation of a protocol-based follow-up program for heart failure patients on readmission and mortality rates and quality of life.
MethodsA quasi-experimental study was performed, with a prospective registry of 50 consecutive patients discharged after hospitalization for acute heart failure. The study group was followed by a cardiologist at days 7-10 and the first, third, sixth and 12th month after discharge, with predefined procedures. The control group consisted of patients hospitalized for heart failure prior to implementation of the program and followed on a routine basis.
ResultsNo significant differences were observed between the two groups regarding mean age (67.1±11.2 vs. 65.8±13.4 years, p=0.5), NYHA functional class (p=0.37), or median left ventricular ejection fraction (27% [19.8-35.3] vs. 29% [23.5-40]; p=0.23) at discharge. Mean follow-up after discharge was similar (11±5.3 vs. 10.9±5.5 months, p=0.81).
The protocol-based follow-up program was associated with a significant reduction in all-cause readmission (26% vs. 60%, p=0.003), heart failure readmission (16% vs. 36%, p=0.032), and mortality (4% vs. 20%, p=0.044). In the study group there was a significant improvement in all quality of life measures (p<0.001).
ConclusionA protocol-based follow-up program for patients with heart failure led to a significant reduction in readmission and mortality rates, and was associated with better quality of life.
Os doentes com insuficiência cardíaca (IC) apresentam taxas elevadas de reinternamento e mortalidade, tornando necessária a implementação de medidas que conduzam à sua redução. Avaliou-se o impacto da implementação de um programa de seguimento estruturado de doentes com IC nas taxas de reinternamento e mortalidade e na qualidade de vida.
MétodosEstudo quasi-experimental, de registo prospetivo, que incluiu 50 doentes consecutivos com alta após internamento por insuficiência cardíaca aguda. Os doentes iniciaram seguimento protocolado após alta, por cardiologista, com consulta aos 7-10 dias, 1, 3, 6 e 12 meses, com procedimentos pré-definidos. O grupo-controlo foi constituído por doentes internados por insuficiência cardíaca previamente à implementação do programa, seguidos após a alta em consultas de rotina.
ResultadosNão houve diferenças entre ambos os grupos no respeitante à idade média (67,1±11,2 versus 65,8±13,4 anos; p=0,5), classe funcional da NYHA (p=0,37) e mediana da fração de ejeção do ventrículo esquerdo [27% (19,8-35,3) versus 29% (23,5-40); p=0,23] à data da alta; o tempo de seguimento médio foi idêntico (11±5,3 versus 10,9±5,5 meses; p=0,81).
O seguimento protocolado associou-se a redução significativa das taxas de reinternamento por qualquer causa (26% versus 60%, p=0,003), reinternamento por insuficiência cardíaca (16% versus 36%, p=0,032) e mortalidade total (4% versus 20%, p=0,044). No grupo em estudo verificou-se melhoria significativa em todos os parâmetros de qualidade de vida (p<0,001).
ConclusãoUm programa de seguimento protocolado de doentes com insuficiência cardíaca permitiu redução significativa nas taxas de reinternamento e mortalidade e associou-se a melhoria da qualidade de vida.
Heart failure (HF) is an important public health problem due to its high prevalence and impact on patients’ quality of life and survival.1–11
In Europe and the USA, the estimated prevalence of HF is 1-2% in adults,1 and a 10-15% increase in the number of affected individuals is projected for the next 10-15 years, reflecting the aging of the population (mainly due to general improvements in health care), the impact of risk factors on the genesis of the syndrome, and the role of comorbidities, particularly in the elderly.2–4 In Portugal, the estimated prevalence of HF is 4.4%, reaching 8% in the seventh decade of life, a higher prevalence than the European average.4–6
Despite the therapeutic advances achieved in recent decades, especially with respect to reductions in sudden cardiac death,7 the mortality attributed to HF remains high.8,9 This is especially true following hospitalization due to decompensated HF, when reported mortality is 17-24% during the year after discharge.10,11
The importance of hospitalization for HF is due not only to the associated mortality, but also to the high readmission rate,12 which imposes a significant economic burden on the health system – 80% of the costs related to the syndrome2 – and poor quality of life for patients with HF.
The readmission rate is particularly high in the vulnerable phase (the first months after discharge), with one-fourth of patients being readmitted in the first month after discharge and two-thirds in the following year.10,13 The transition phase (pre- and post-discharge) is therefore of particular importance in terms of care, planning and follow-up, since one of the main factors responsible for early readmission is lack of coordination of care after hospital discharge.6,14
Several post-discharge follow-up strategies have been proposed, although not all have shown a significant impact on outcomes.15–17 Structured follow-up programs based on hospital consultations are often associated with a reduction in readmissions during the first year (relative risk reduction [RRR] of 19-30%),15,16,18 and also in the risk of death.15–17 The inclusion of patients with HF in such programs is therefore recommended by the European Society of Cardiology (ESC).19 However, much of the evidence on which this recommendation is based derives from a time when some contemporary therapies, particularly cardiac resynchronization therapy (CRT) and implantable cardioverter defibrillators (ICD), were not widely available.15–17 There is also no evidence on the impact of the implementation of such programs in Portugal.
The objective of this single-center study was to assess the results of implementing a structured follow-up program for HF patients on readmission and mortality rates and on quality of life, after an episode of hospitalization due to the syndrome.
MethodsDesign and populationThis was a quasi-experimental design study carried out in a single center (the cardiology department of Santa Maria University Hospital, Lisbon, Portugal).
The study population consisted of 50 consecutive patients admitted for acute heart failure (AHF), defined as new-onset AHF or decompensated chronic heart failure, to the general cardiology ward (index hospitalization), who were discharged after the implementation of a protocol-based follow-up program (beginning in April 2016). The diagnosis of HF was established according to the ESC guidelines, through the identification of symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality, resulting in reduced cardiac output and/or elevated intracardiac pressures.19
The control group consisted of patients selected from a cohort hospitalized for AHF in the same cardiology ward immediately before the beginning of the program (from October 2014 to April 2016). Patients from both cohorts (study group and control group) were classified according to New York Heart Association functional class (NYHA) at discharge (NYHA I vs. NYHA II vs. NYHA III or IV), left ventricular ejection fraction (LVEF) (tertiles), and age (tertiles). For each of the patients in the study group a patient from the control cohort with similar scores in each of the three variables was randomly selected.
Demographic, clinical, laboratory, echocardiographic, electrocardiographic and therapeutic data regarding the index hospitalization and the follow-up period after discharge were collected for the study group and for all patients who constituted the control cohort. Quality of life was assessed at discharge and at six-month follow-up using the validated Portuguese version of the Kansas City Cardiomyopathy Questionnaire (KCCQ).20
Interventions differentiating the study groupThe main differentiating intervention in the protocol-based follow-up program was consultations by a cardiologist at days 7-10 and the first, third, sixth and 12th month after discharge (and additionally, whenever considered necessary) with pre-specified procedures. These included:
- (a)
Clinical assessment aimed at identifying signs or symptoms of HF decompensation, residual congestion or low cardiac output;
- (b)
Laboratory assessment, including monitoring of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) level, end-organ dysfunction, and development of common comorbidities in HF patients (diabetes, chronic pulmonary disease, dyslipidemia, thyroid dysfunction, anemia, iron deficiency)19;
- (c)
Electrocardiogram at every visit, and transthoracic echocardiogram between the third and sixth months and every 12 months of follow-up; when considered necessary, cardiac magnetic resonance imaging study was requested;
- (d)
Assessment of adherence and tolerance to therapy;
- (e)
Individualized titration of therapy in accordance with the ESC guidelines19;
- (f)
Patient education regarding self-care, lifestyle modifications, and management of HF decompensation.19
The primary outcome was all-cause readmission. HF readmission, death and the composite endpoint of all-cause readmission or death were secondary outcomes.
In the structured follow-up program group, changes in quality of life parameters was also considered a secondary outcome.
Also, in the study group, LVEF change was assessed in the subgroup of patients with LVEF <50%, and the prescription rate of neurohormonal antagonists and changes in their respective doses during follow-up was assessed in the subgroup of patients with HF with reduced LVEF (HFrEF) (LVEF <40%).
Statistical analysisAssuming that the estimated annual rate of all-cause readmission would be 65% in the control group and 35% in the study group (based on HF populational studies10,13 and HF post-discharge programs studies,15 respectively), it was estimated that 42 patients would need to be followed in each group for 12 months to provide the study with a power of 80% to detect a significant relative reduction in the risk of all-cause readmission in the follow-up program group, at an overall two-sided alpha level of 0.05.
The statistical analysis was performed using IBM SPSS® Statistics version 20 (Chicago, IL, USA). Categorical variables are reported as absolute number and percentage and continuous variables are reported as mean and standard deviation or median and interquartile range. The impact of inclusion in the structured follow-up program on readmission and mortality rates was assessed using Cox regression and Kaplan-Meier survival analysis. Wilcoxon's test was used to assess the impact of the follow-up program on quality of life, doses of neurohormonal antagonists and LVEF. Differences between the groups regarding demographic, clinical and therapeutic data were established using the Mann-Whitney, Student's t, chi-square, one-way ANOVA and Fisher's exact tests. p values of <0.05 were considered to indicate statistical significance.
Ethical considerationsThe study was approved by the local ethics committee and by the national Data Protection Authority. Patient confidentiality was ensured through anonymization of the collected data. All study procedures were carried out in accordance with the ethical principles expressed in the 2013 revision of the Declaration of Helsinki.21
ResultsPopulation characteristicsThe first patient was enrolled in the protocol-based follow-up program in April 2016 and the 50th patient in November 2017. The mean follow-up was 11±5.3 months in the study group and 10.9±5.5 months in the control group (p=0.81).
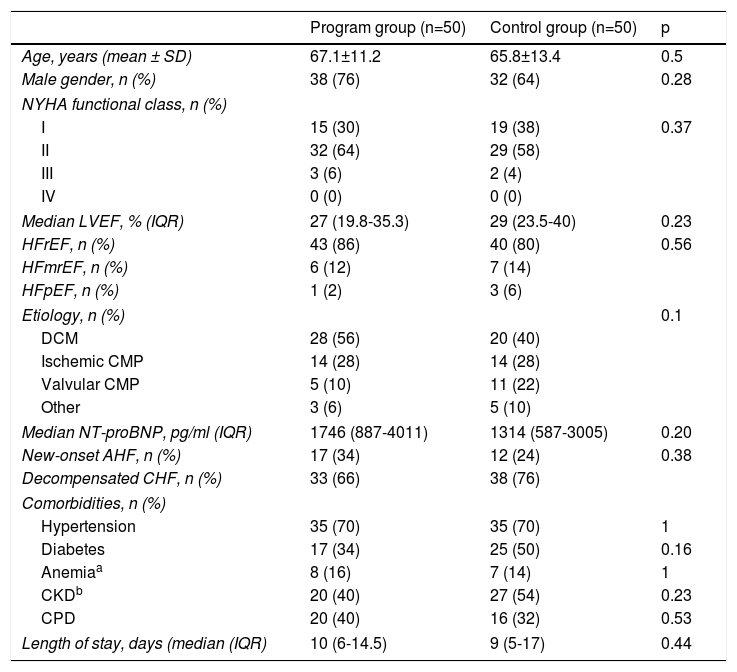
Patients’ demographic and clinical characteristics at discharge are described in Table 1.
Population characteristics at discharge.
| Program group (n=50) | Control group (n=50) | p | |
|---|---|---|---|
| Age, years (mean ± SD) | 67.1±11.2 | 65.8±13.4 | 0.5 |
| Male gender, n (%) | 38 (76) | 32 (64) | 0.28 |
| NYHA functional class, n (%) | |||
| I | 15 (30) | 19 (38) | 0.37 |
| II | 32 (64) | 29 (58) | |
| III | 3 (6) | 2 (4) | |
| IV | 0 (0) | 0 (0) | |
| Median LVEF, % (IQR) | 27 (19.8-35.3) | 29 (23.5-40) | 0.23 |
| HFrEF, n (%) | 43 (86) | 40 (80) | 0.56 |
| HFmrEF, n (%) | 6 (12) | 7 (14) | |
| HFpEF, n (%) | 1 (2) | 3 (6) | |
| Etiology, n (%) | 0.1 | ||
| DCM | 28 (56) | 20 (40) | |
| Ischemic CMP | 14 (28) | 14 (28) | |
| Valvular CMP | 5 (10) | 11 (22) | |
| Other | 3 (6) | 5 (10) | |
| Median NT-proBNP, pg/ml (IQR) | 1746 (887-4011) | 1314 (587-3005) | 0.20 |
| New-onset AHF, n (%) | 17 (34) | 12 (24) | 0.38 |
| Decompensated CHF, n (%) | 33 (66) | 38 (76) | |
| Comorbidities, n (%) | |||
| Hypertension | 35 (70) | 35 (70) | 1 |
| Diabetes | 17 (34) | 25 (50) | 0.16 |
| Anemiaa | 8 (16) | 7 (14) | 1 |
| CKDb | 20 (40) | 27 (54) | 0.23 |
| CPD | 20 (40) | 16 (32) | 0.53 |
| Length of stay, days (median (IQR) | 10 (6-14.5) | 9 (5-17) | 0.44 |
Estimated glomerular filtration rate <60 ml/min/1.73 m2 (calculated by the Chronic Kidney Disease Epidemiology Collaboration formula).
AHF: acute heart failure; CHF: chronic heart failure; CKD: chronic kidney disease; CMP: cardiomyopathy; CPD: chronic pulmonary disease; DCM: dilated cardiomyopathy; HFmrEF: heart failure with mid-range ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association.
The mean age of the follow-up program group was 67.1±11.2 years and 38 patients (76%) were male. Most patients were in NYHA I (30%) or II (64%) at discharge; all patients were in NYHA III (52%) or IV (48%) on admission (index hospitalization). The median LVEF documented at discharge was 27% (19.8-35.3), and 43 (86%) patients had HFrEF. There were no significant differences between the two groups regarding age, NYHA or LVEF.
The most frequent HF etiology in both groups was idiopathic dilated cardiomyopathy (56% vs. 40%), followed by ischemic heart disease (28% vs. 28%). Overall, 70% of the patients had a history of hypertension, making it the most common comorbidity in both groups.
Median length of stay and median plasma NT-proBNP at discharge did not differ significantly between groups.
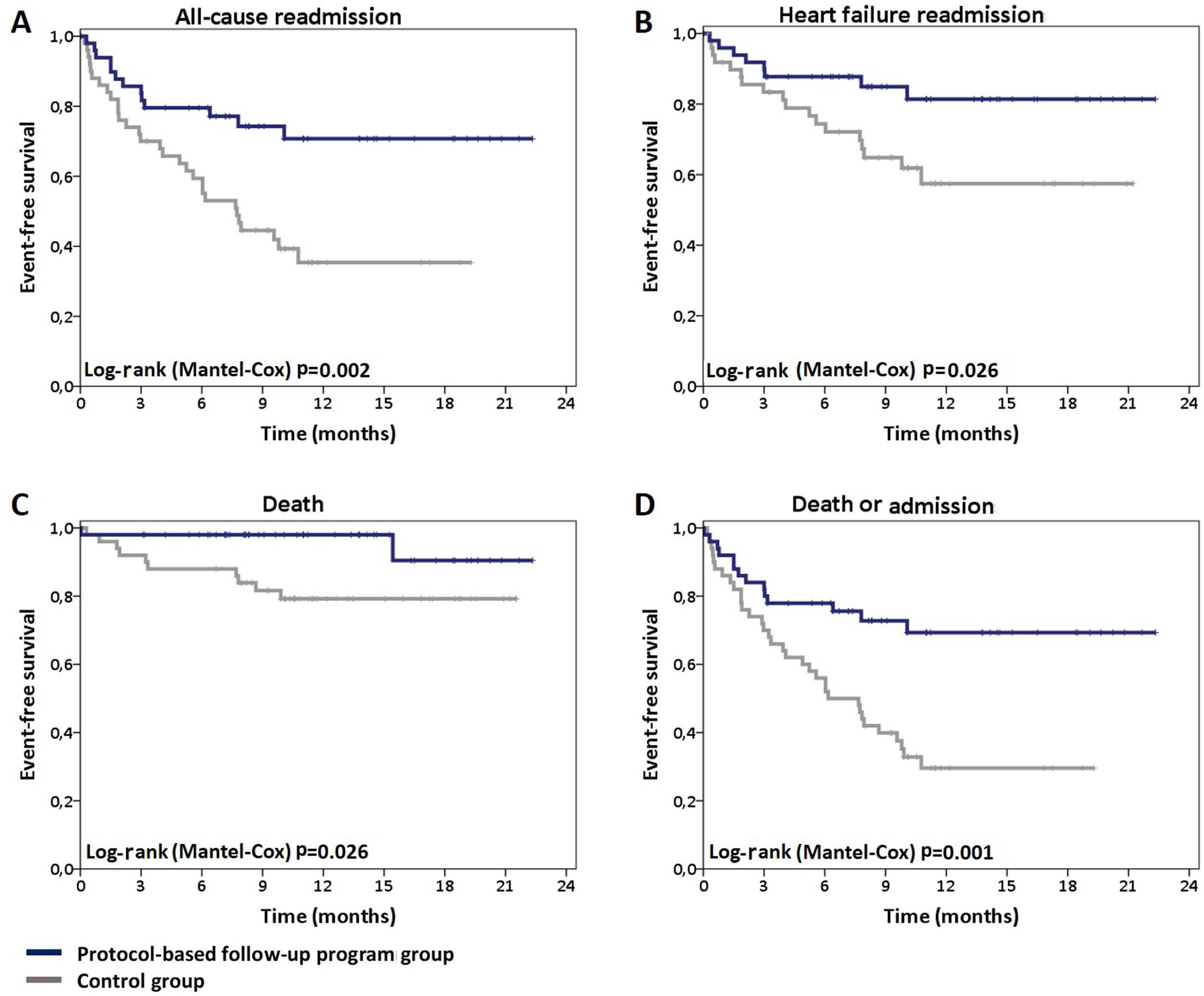
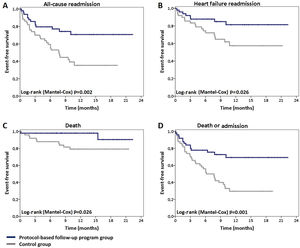
Mortality and readmission ratesCompared to patients in the control group, those included in the structured follow-up program showed a significant reduction in all-cause readmissions (26% vs. 60%; hazard ratio [HR] 0.38 [0.2-0.73]; p=0.003) (Figure 1A). The RRR was 56.7% and the number needed to treat (NNT) was 2.91. A similar benefit was achieved in HF readmission (RRR: 64.4%; NNT: 3.45) (Figure 1B). Eighteen (36%) patients in the control group were hospitalized due to AHF. Implementation of the protocol-based follow-up program led to an HF readmission rate of 16% (eight patients) (HR 0.4 [0.17-0.92]; p=0.032).
Mortality was significantly lower in patients enrolled in the follow-up program (4% vs. 20%; HR 0.21 [0.05-0.96]; p=0.044). The RRR was 80% and the NNT was 6.25 (Figure 1C).
During follow-up two (4%) patients in the study group died, one due to right ventricular failure in the immediate postoperative period following cardiac surgery, and the other due to sudden death. The latter patient underwent ICD implantation during the index hospitalization and sudden death occurred in the first week after hospital discharge, before the first protocol-based follow-up visit. Autopsy was not performed and interrogation of the ICD showed no dysrhythmia or evidence of device dysfunction. In the control group, two (4%) patients died during HF hospitalization, and four (8%) during hospitalization due to other causes.
The secondary outcome of death or all-cause hospitalization was significantly less frequent in the follow-up program group (28% vs. 68%; HR 0.36 [0.19-0.67]; p=0.001), with an NNT of only 2.5 (Figure 1D).
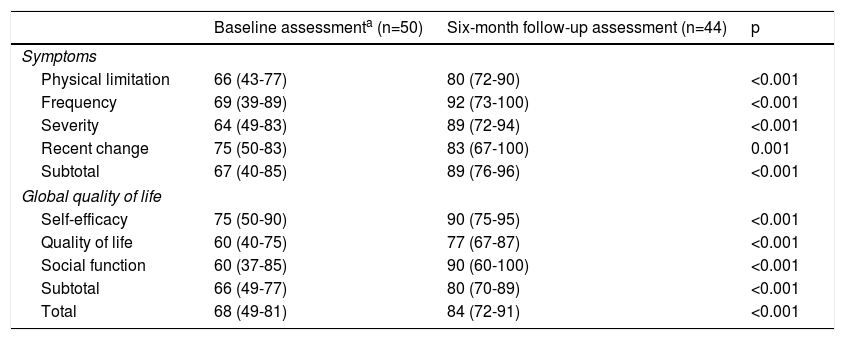
Quality of life and functional classIn the study group, a significant improvement was observed in all KCCQ domains, especially in the overall summary scores for symptoms (67% vs. 89%, p<0.001) and quality of life (66% vs. 80%, p<0.001) (Table 2).
Quality of life in the study group as assessed by the Kansas City Cardiomyopathy Questionnaire (validated Portuguese version).
| Baseline assessmenta (n=50) | Six-month follow-up assessment (n=44) | p | |
|---|---|---|---|
| Symptoms | |||
| Physical limitation | 66 (43-77) | 80 (72-90) | <0.001 |
| Frequency | 69 (39-89) | 92 (73-100) | <0.001 |
| Severity | 64 (49-83) | 89 (72-94) | <0.001 |
| Recent change | 75 (50-83) | 83 (67-100) | 0.001 |
| Subtotal | 67 (40-85) | 89 (76-96) | <0.001 |
| Global quality of life | |||
| Self-efficacy | 75 (50-90) | 90 (75-95) | <0.001 |
| Quality of life | 60 (40-75) | 77 (67-87) | <0.001 |
| Social function | 60 (37-85) | 90 (60-100) | <0.001 |
| Subtotal | 66 (49-77) | 80 (70-89) | <0.001 |
| Total | 68 (49-81) | 84 (72-91) | <0.001 |
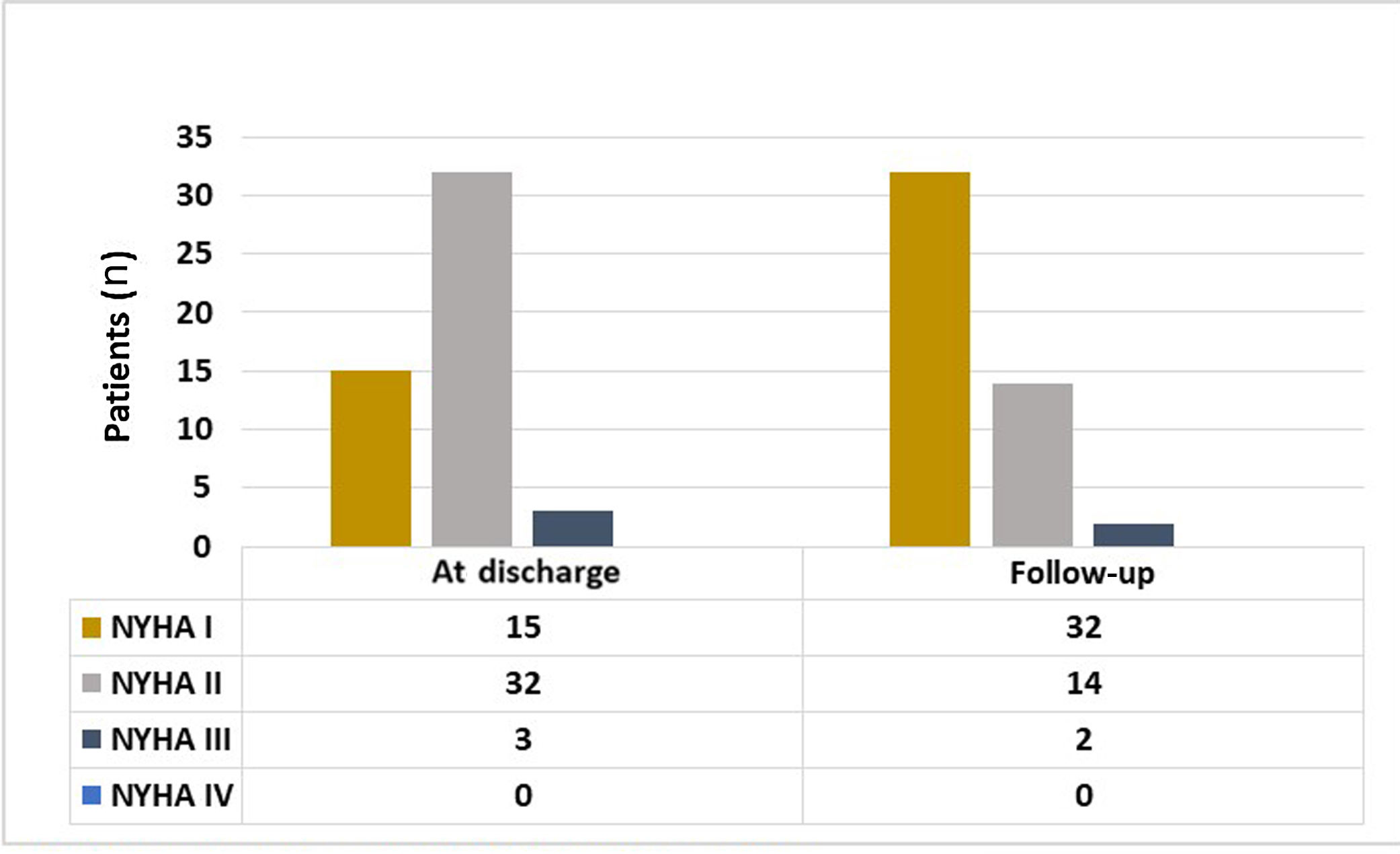
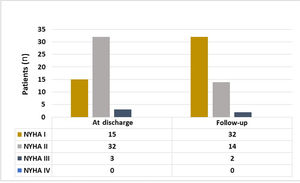
Parallel to the improvement in the KCCQ symptoms domain reported by the patients, there was a significant improvement in NYHA class documented by the cardiologist in the last follow-up visit compared to NYHA class at discharge. In the last clinical assessment performed most patients were in NYHA I (64% vs. 30%, p<0.001) (Figure 2).
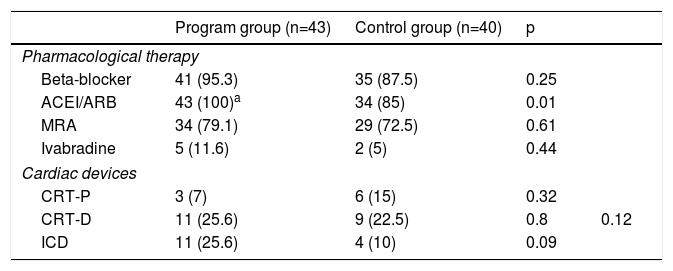
Therapy with neurohormonal antagonistsAs stated above, the majority of patients in both groups presented HFrEF. Table 3 describes ongoing therapy (neurohormonal antagonists and cardiac devices) at the time of the last clinical assessment.
Pharmacological therapy and cardiac devices at last clinical assessment.
| Program group (n=43) | Control group (n=40) | p | ||
|---|---|---|---|---|
| Pharmacological therapy | ||||
| Beta-blocker | 41 (95.3) | 35 (87.5) | 0.25 | |
| ACEI/ARB | 43 (100)a | 34 (85) | 0.01 | |
| MRA | 34 (79.1) | 29 (72.5) | 0.61 | |
| Ivabradine | 5 (11.6) | 2 (5) | 0.44 | |
| Cardiac devices | ||||
| CRT-P | 3 (7) | 6 (15) | 0.32 | |
| CRT-D | 11 (25.6) | 9 (22.5) | 0.8 | 0.12 |
| ICD | 11 (25.6) | 4 (10) | 0.09 | |
This group includes three patients treated with sacubitril/valsartan.
Data are reported as total number and percentage. ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CRT-D: cardiac resynchronization therapy with cardioverter-defibrillator; CRT-P: cardiac resynchronization therapy-pacemaker; ICD: implantable cardioverter-defibrillator; MRA: mineralocorticoid receptor antagonist.
The rate of prescription of beta-blockers, mineralocorticoid receptor antagonists (MRAs) and ivabradine did not differ between the two groups. However, a significantly higher rate of prescription of angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) was observed in patients included in the follow-up program group compared to controls (100% vs. 85%, p=0.01). There were no significant differences regarding CRT or ICD implantation rates (Table 3).
Considering the whole population, there were no significant differences in diuretic therapy prescription between groups (44 vs. 47 patients, p=NS). All these patients were medicated with loop diuretics; four patients in the follow-up program group and six patients in the control group were under an association of a loop plus a thiazide diuretic (p=NS).
There were no differences in myocardial revascularization procedures, either percutaneous angioplasty (6% vs. 8%, p=NS), or coronary artery bypass graft surgery (2% in both groups, p=NS). Rates of aortic (6% vs. 8%, p=NS) and mitral (6% vs. 4%, p=NS) valve interventions (percutaneous or surgical) were also similar in both groups.
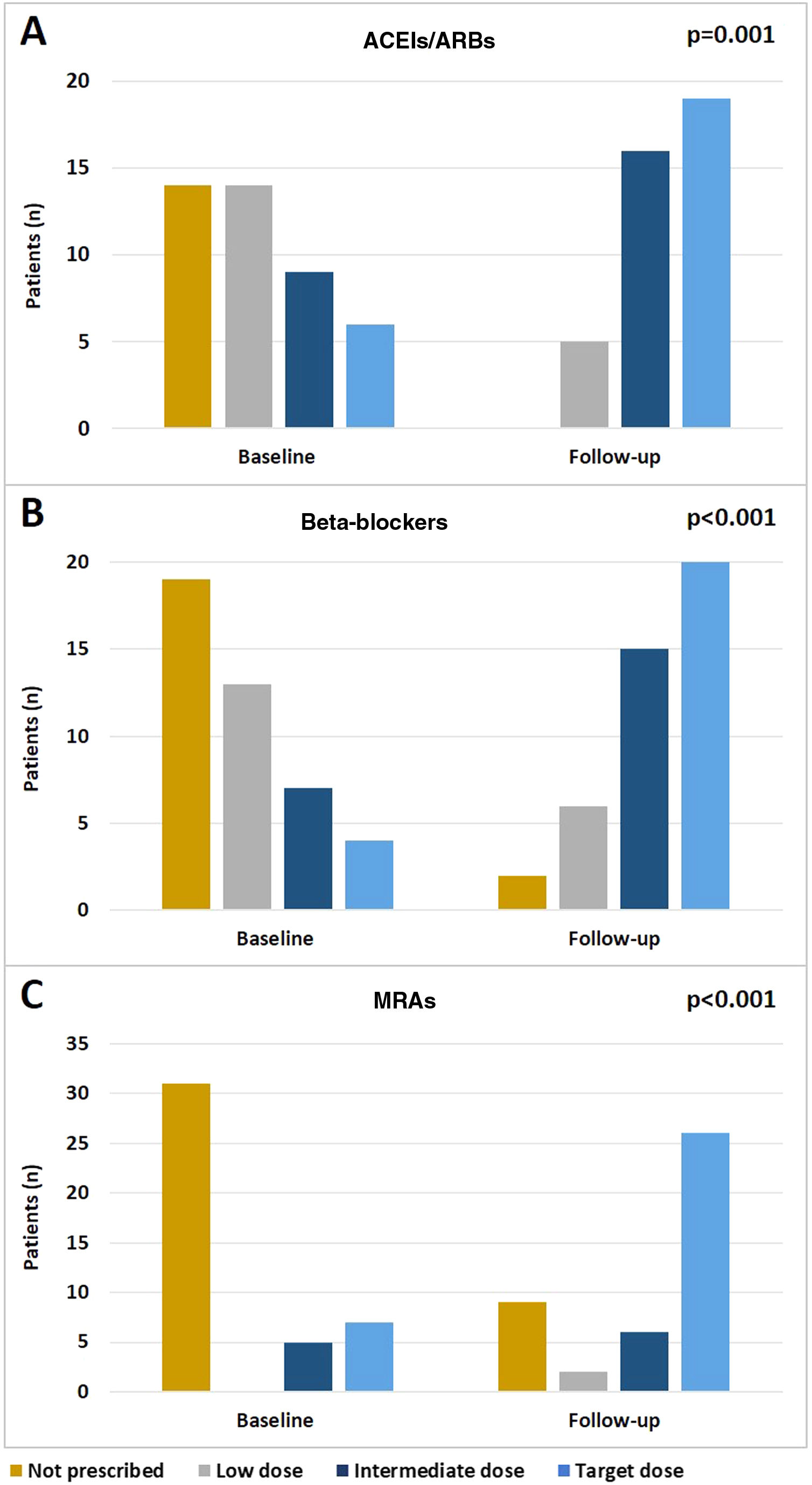
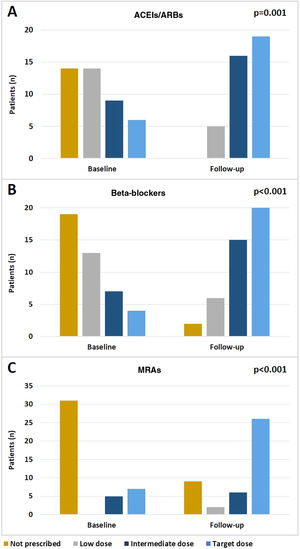
In the study group there was an effective optimization of treatment during follow-up, including up-titration of recommended drugs. Significant increases in the doses of ACEIs/ARBs (p=0.001), beta-blockers (p<0.001) and MRAs (p<0.001) (Figure 3A-C, respectively) were observed between admission and the last follow-up visit.
In the subgroup of patients with HFrEF or HF with mid-range ejection fraction enrolled in the protocol-based follow-up program (n=49), there was a significant improvement in LVEF during follow-up (27% [19.8-35.3] vs. 39.5% [29.5-50]; p<0.001).
DiscussionThe impact of hospitalizations on the natural history of HF is illustrated by the frequently reported increase in mortality during the period that follows a hospital admission due to the syndrome. In this period, not only does the mortality risk increase, but a vicious cycle leading to further hospitalizations is triggered.10,22 This is of major importance at a time when, despite all the therapeutic advances, HF readmission rates continue to increase.9,23 In Portugal, according to the latest published report of the National Program for Cerebro-Cardiovascular Diseases,9 the number of hospital admissions due to HF episodes was 19 434 in 2015 (2365 deaths), an increase of 4000 episodes compared to 2011 (15 583 hospitalizations, 2046 deaths), making it imperative to establish strategies that may lead to a reduction in the (re)hospitalization rate, and consequently in mortality, in these patients.6
In this study we present the characteristics and results of a protocol-based follow-up program conducted at the cardiology department of a European tertiary hospital. Prior to the implementation of the program, on the basis of the frequency of major events in the control group, readmission (60%) and mortality (20%) after hospital discharge in patients admitted for an HF episode were similar to or even higher than those reported in the literature.10,11,13 The HF readmission rate in our control population was similar to that reported in published data on Portuguese cohorts (36% vs. 30.5%), although mortality was lower (20% vs. 34.3%).24 However, implementation of the structured follow-up program led to a marked reduction in all-cause readmission (absolute risk reduction of 40%), and also to a considerable though smaller reduction in readmissions due to decompensated HF (absolute risk reduction of 20%). Concomitantly a significant reduction in mortality was obtained (from 20% to 4%).
The benefit of including patients in the follow-up program is evident when the secondary endpoint (death or readmission) is analyzed, as the reduction of major events resulted in an NNT of 2.5, which is, interestingly, a better result than that reported for conventional HF therapies.25–32
Unlike various other follow-up or monitoring strategies designed to reduce hospitalizations and mortality in the HF population, such as telemonitoring or follow-up programs based on telephone contacts,33,34 protocol-based follow-up programs have demonstrated consistent benefits. These benefits do, however, not reach the magnitude reported in this study.15–17 One possible reason for this difference may be that most of the beneficial evidence from these follow-up programs goes back to a time when some of the current HF treatment options were not yet available.
Additionally, the magnitude of benefit achieved may be related to the intrinsic characteristics of our protocol-based follow-up program. At first, during hospitalization and in the pre-discharge period, a careful management plan was set up according to the patients’ characteristics and needs. The follow-up program after discharge was based on face-to-face consultations and on predefined procedures (see Methods) with demonstrated benefits in event reduction, favoring a holistic approach based on international guidelines.19
It is important to highlight the frequency of the consultations, with particular emphasis on the visits at days 7-10 and at one month after discharge. Early post-discharge visits have important benefits for reducing readmission and mortality, not only during the first month after discharge, but also thereafter.18,22,35 This is related to the importance of the hospital-home transition and to the problems that patients usually face, including difficulties in managing medication, unfamiliarity with the necessary changes in lifestyle, lack of knowledge about their disease, and management of worsening symptoms. The role of the early post-discharge consultations focuses on helping with these problems and, when necessary, on therapeutic optimization.
At each visit, the presence of symptoms or signs indicative of decompensation was carefully assessed and appropriate therapeutic measures were taken. This may have contributed decisively to the marked reduction in HF readmission. However, it should be noted that much of the benefit derived from this program was seen in the reduction in all-cause readmissions. To accomplish this, it was crucial to pay particular attention to the monitoring and management of comorbidities, which have a significant impact on HF patients’ prognosis, as most hospitalizations in this population are for non-cardiac causes.23
It is also worth emphasizing the effect of the program on the up-titration of neurohormonal antagonist doses, as the vast majority of patients with HFrEF were treated with beta-blockers (95.3%), ACEIs/ARBs (100%), and MRAs (79.1%), and in many patients target doses were achieved (46.5%, 47.5% and 60.5%, respectively). The frequency of hospital visits and assessment of therapeutic tolerance may have played a part in this achievement. However, although optimization of pharmacologic therapy may have had an important role in the observed marked reduction of events during follow-up, it should also be noted that the number of patients treated with these drugs in the control group was also high, even higher than previously reported in Portuguese HF cohorts.24 The rate of CRT and ICD implantation was similar between the two groups and similar to that reported in European cohorts.10 This finding supports the added benefit of this follow-up program with holistic interventions on top of the benefit associated with medical and device HF therapies.
The follow-up program was also associated with significant improvements in both NYHA functional class and quality of life and symptoms as assessed by the KCCQ. The scores obtained in both domains at discharge were similar to those in the literature (66% and 67% vs. 56% and 63%, respectively),36 whereas at the sixth month of follow-up the scores reported herein were significantly higher (80% and 89%, respectively). Particular attention should be given to the ‘self-efficacy’ sub-domain, which assesses patients’ perceived ability to manage their own symptoms. The population included in the follow-up program had a relatively high self-efficacy score (75%) at the time of discharge, which may be due to the education on patient self-care provided during hospital stay. Nevertheless, a significant additional improvement (90%) was observed during follow-up, demonstrating the effectiveness of the HF self-care management and health-related education reinforcement carried out at each consultation. This may also have accounted for the reduction in major adverse events.
To our knowledge this is the first study reporting the efficacy of a protocol-based follow-up program in reducing readmission and mortality in a Portuguese population with HF, filling an apparent evidence gap6,37 and, we hope, perhaps helping to encourage the development of this type of program in other hospital centers.
LimitationsThe data reported should be interpreted in the light of certain limitations, particularly the fact that this was not a randomized controlled study and that the sample size was small (the first 50 patients enrolled and followed by protocol). Besides, only patients admitted to the cardiology department were included, which naturally entails selection bias, resulting in a population with a higher proportion of patients with HFrEF and younger than those reported in studies that included patients admitted to internal medicine departments.24 Additionally, cost-effectiveness analysis was not performed, so it is not possible to determine whether the reduction in events during the follow-up period was accompanied by a reduction in costs attributed to HF. However, as significant reductions in the admission rate were obtained, this probably translated into reductions in costs related to the syndrome and to associated comorbidities. Finally, the study only included patients with a recent admission due to AHF, so it is not possible to assess the impact of this follow-up program on stable patients with chronic HF but with no previous recent HF-related hospital admissions. As reported, most adverse events in HF patients occur in the first year after hospital discharge.10,11,13 In fact, despite the small number of patients who completed more than 12 months of follow-up in our population, through the data obtained from the Kaplan-Meier survival curves (Figure 1A-D), it may be assumed that the incidence of major adverse events during the second year of follow-up would have been low, suggesting a reduced benefit of this type of program in stable patients, as previously suggested.38,39 However, we consider that more evidence is needed in order to establish the ideal duration of this type of program, according to patients’ clinical profile and disease progression.
ConclusionsDespite all the advances achieved in the treatment of patients with HF – including drugs designed to modify prognosis, cardiac devices and management of comorbidities – morbidity and mortality attributed to the syndrome remain high. Structured follow-up programs may have a key role in the management of these patients.
This study reports the results of the implementation of such a program in the cardiology department of a tertiary hospital, and is the first to document its benefits in a Portuguese population with HF. The program was associated with marked reductions in HF readmission, all-cause readmission and mortality, and with significant improvements in functional class and in patients’ self-reported quality of life.
The results support the need for investment in this type of program as a means to improve the prognosis of patients with HF, and consequently to reduce the burden attributed to the condition.
Authors’ contributionsJRA and DB designed the protocol and wrote the manuscript. JRA performed the statistical analysis. All authors contributed similarly to data collection, recording and interpretation, reviewed the article and gave their final approval for the version to be published.
SponsorshipThe study did not receive any sponsorship.
Conflicts of interestThe authors have no conflicts of interest to declare.