We report a case of a 73-year-old female patient admitted to the surgical department for a splenic abscess. She had a history of a mechanical aortic valve implanted two years earlier. During the diagnostic work-up, the patient underwent a transesophageal echocardiogram that revealed the presence of multiple paravalvular abscesses, establishing the diagnosis of prosthetic valve endocarditis. A few days later, the echocardiogram was repeated due to a new-onset systolic-diastolic murmur. A large pseudoaneurysm and significant periprosthetic regurgitation were now noted and the patient was referred for cardiac surgery. The microbiologic exam revealed the presence of Streptococcus milleri, usually found in the gastrointestinal flora and a known pathogenic agent of endocarditis. Interestingly, the patient had had a foreign body (bone fragment) removed from her esophagus a few weeks earlier, which was the probable portal of entry for this infective endocarditis.
Os autores reportam o caso de uma doente de 73 anos de idade, do sexo feminino, admitida no Serviço de Cirurgia Geral por um abcesso esplénico. A doente tinha antecedentes de uma prótese mecânica em posição aórtica implantada dois anos antes. Durante o estudo diagnóstico foi realizado um ecocardiograma transesofágico que revelou a presença de múltiplos abcessos periprotésicos que permitiram estabelecer o diagnóstico de endocardite de prótese. Alguns dias depois a doente repetiu o exame por sopro sisto-diastólico de novo. Neste estudo destacava-se agora a presença de um volumoso pseudoaneurisma e uma regurgitação periprotésica significativa, pelo que a doente foi orientada para cirurgia cardíaca urgente. O estudo microbiológico revelou a presença de Streptococcus milleri, um constituinte normal da flora gastrointestinal e um agente patogénico já conhecido de endocardite infecciosa. Curiosamente, a doente tinha antecedentes de remoção de um corpo estranho alojado no esófago (um fragmento ósseo) algumas semanas antes, e que foi a provável porta de entrada para a endocardite infecciosa.
Infective endocarditis (IE) is a relatively common disease with an estimated in-hospital mortality of 15–20%.1,2 Currently, the most common agent of IE is Staphylococcus aureus, accounting for approximately one-third of cases,3 followed by Streptococcus viridans. This reflects a shift in the epidemiology of IE, mainly driven by medical progress and increased health care contact, and a consequent rise in the incidence of Staphylococcus bacteremia.3 It also reflects changes in the characteristics of patients with IE, with an increasing incidence of patients presenting with immunodeficiency and/or cardiac prosthetic devices.3 While multiple factors contribute to the pathophysiology of IE, the presence of bacteremia is an essential condition for the occurrence of an infection in cardiac structures.4 Prophylactic antibiotic therapy is therefore recommended for high-risk patients before some medical procedures.5 Although not all procedures are considered in these recommendations, as discussed later, it should be kept in mind that, independently of its source, the occurrence of bacteremia is itself a risk factor for the occurrence of IE. In view of this consideration we report a case of a patient with prosthetic valve endocarditis following the removal of a foreign body from the esophagus.
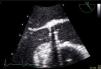
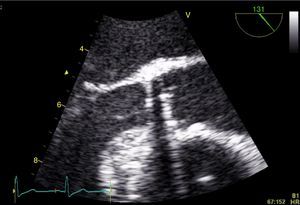
Case reportA 73-year-old female patient presented to the emergency department complaining of asthenia, weight loss (>10% of initial body weight) and fever (38–38.5°C) during the last four weeks. She had a history of a single-disc mechanical aortic valve implanted two years earlier due to severe aortic stenosis. She was taking warfarin and was doing well in the follow-up. After an initial diagnostic work-up, in which no infectious focus was identified, the patient underwent transthoracic and transesophageal echocardiograms. Both exams showed a normally functioning mechanical prosthesis, normal left ventricular systolic function, and no vegetations or images compatible with abscesses or other paravalvular complications (Figure 1).
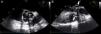
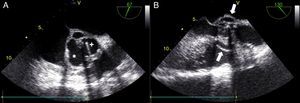
One month later, the patient presented again in the emergency department complaining of abdominal pain and persistent asthenia and fatigue. An abdominal computed tomography scan showed a large splenic mass and an exploratory laparotomy was performed. Intraoperatively, an extensive abscess was noted which extended to the diaphragm and ipsilateral pleural cavity. The spleen was removed and the material collected for microbiological analysis. Given this setting, and to exclude potential embolic sources, echocardiographic assessment was repeated. The transesophageal echocardiogram showed multiple abscesses (Figure 2A, asterisk) surrounding the single-disc mechanical aortic prosthesis (Figure 2A, cross). There appeared to be no communication between the abscesses and the cardiac cavities, thus excluding the possibility of a pseudoaneurysm (Figure 2B, white arrow) and there were no signs of prosthetic dysfunction. The patient began appropriate antibiotic therapy and was referred for surgery.
Transesophageal echocardiogram in mid-esophageal short- (A) and long-axis (B) views of the aortic prosthesis, showing two abscesses (A, asterisk) surrounding the single-disc mechanical prosthetic valve (A, cross). There appears to be no rupture from these abscesses (B, white arrow) to the left ventricular outflow tract.
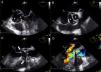
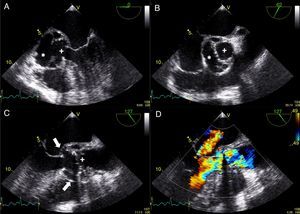
While awaiting surgery a new-onset systolic-diastolic murmur was noted. The echocardiogram performed at that time revealed marked paravalvular involvement of the prosthetic valve with multiple larger abscesses (Figure 3A and 3B, asterisks) surrounding the anterior, posterior and right portions of the prosthesis (Figure 3A and 3B, cross) which communicated with the left ventricular outflow tract, forming a pseudoaneurysm that extended 3 cm above the aortic valve plane (Figure 3C, white arrows). Severe periprosthetic regurgitation was also noted (Figure 3D) and the patient underwent cardiac surgery. Meanwhile, microbiological study revealed the presence of Streptococcus milleri sensitive to the empirically started antimicrobial therapy. Interestingly, the patient had a history of ingestion of a bone fragment that had been removed from the esophagus by upper gastrointestinal endoscopy 2–3 weeks before the beginning of the constitutional symptoms. This event was the most probable portal of entry for the bacteremia. Unfortunately, the patient died in the perioperative period due to surgical complications.
Transesophageal echocardiogram showing multiple abscesses (asterisk) surrounding the single-disc mechanical prosthetic valve (cross). These abscesses had ruptured to the left ventricular outflow tract forming a pseudoaneurysm (white arrow) extending above the aortic valve plane. Severe periprosthetic regurgitation and mild to moderate mitral regurgitation were also noted (D). The images were obtained in mid-esophageal view at 0° (A); short-axis view (B); and long-axis view without (C) and with (D) color flow Doppler.
Herein, we report a case of a fatal IE with extensive paravalvular complications in a patient with a mechanical prosthetic aortic valve, occurring some weeks after the removal of a foreign body lodged in the esophagus. While the origin of the bacteremia may be controversial in this case, S. milleri has been identified in the normal gastrointestinal mucosa6 and has been previously described as an uncommon agent of IE.7 In a study comparing the clinical course of IE among Streptococcus species, Lefort et al. reported that the portal of entry was the gastrointestinal system in about 10% of cases caused by S. milleri.7 This information, together with the temporal association between the bone removal and the onset of symptoms, corroborates the idea that the ingestion and removal of the foreign body was the portal of entry for the agent identified in our patient. Additionally, S. milleri has been associated with a particular predisposition to pyogenic infections and abscess formation.8,9 This characteristic may have contributed to the distinct clinical course of our patient's IE, which can be divided into two separate phases. After an initial locally indolent period without evidence of endocarditis or prosthesis dysfunction on the transesophageal echocardiogram, the patient experienced a rapidly evolving pyogenic phase of the disease in spite of appropriate treatment with broad-spectrum antibiotics. These complications ultimately led to systemic and paravalvular complications, with the formation of a pseudoaneurysm and several abscesses that were well documented on the transesophageal echocardiogram. The value of this exam in the diagnosis and management of IE is indisputable, with good sensitivity and specificity for the detection of vegetations and paravalvular complications. However, in the presence of mechanical prosthetic valves, as in the case of our patient, a negative transesophageal echocardiogram significantly decreases the likelihood of IE, but does not completely exclude the diagnosis.10 In such scenarios the clinical course assumes particular importance for a correct diagnosis.10 This may explain the absence of findings suggestive of IE on the patient's first transesophageal echocardiogram.
Finally, while in our patient the foreign body itself could have been responsible for the bacteremia, the question arises of the need for prophylactic antibiotic therapy in medical procedures. In recent years, recommendations for prophylactic antibiotic therapy have changed significantly.11 In fact, in the light of new evidence showing that IE is more likely to occur following bacteremia due to daily routine activities than following medical procedures, recommendations for prophylactic antibiotic therapy are now limited to high-risk patients undergoing dental procedures.5 While there is no compelling evidence supporting prophylaxis in patients undergoing gastrointestinal procedures, there are anecdotal reports supporting this practice based on clinical cases in which the gastrointestinal system was the portal of entry for the IE.12 Irrespective of the recommendations in the guidelines, it should be borne in mind that any condition predisposing to bacteremia is a foundation for the development of IE, particularly in high-risk patients. This recognition may lead to a more careful medical work-up after febrile illness in patients exposed to a potential source of bacteremia.
ConclusionThe present case highlights the variability in the clinical presentation of IE depending on the microbiological agent responsible for the disease, and reinforces the importance of bacteremia in its pathophysiology. Specifically, it illustrates the rapidly progressive course of IE caused by S. milleri, and shows striking images of paravalvular complications in a patient with a mechanical prosthetic aortic valve. This case also emphasizes that transesophageal echocardiography is less accurate in the diagnosis of IE in patients with mechanical prosthetic valves, in whom the clinical scenario may assume a preponderant role.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.