Neutrophil gelatinase-associated lipocalin (NGAL) is an early marker of kidney injury. We sought to assess the prognostic value of this biomarker in patients with stable chronic heart failure (HF).
MethodsWe studied 61 patients with chronic systolic HF who had been receiving optimal medical treatment for at least six months. Biomarkers were measured at baseline and included plasma NGAL, microalbuminuria, serum creatinine, and B-type natriuretic peptide (BNP). Estimated glomerular filtration rate (eGFR) was also calculated. Mean follow-up was 10.6±6.6 months. The primary endpoint was time to first cardiovascular event, defined as a combination of cardiovascular death, HF hospitalization or emergency department visit due to HF. Variables independently related to events were determined using a Cox proportional hazards model.
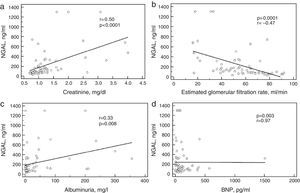
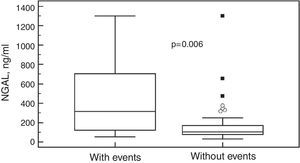
ResultsFifteen (24.6%) patients reached the primary endpoint. Patients with events were more likely to have worse renal function at baseline and also higher NGAL levels (median 316 [interquartile range 122–705] vs. 107 [78–170]; p=0.006). NGAL correlated significantly with creatinine (r=0.50; p<0.0001), albuminuria (r=0.33; p=0.008), and eGFR (r=−0.47; p=0.0001) but not with BNP (r=0.003; p=0.97). The best NGAL cutoff as determined by ROC curve analysis was 179 ng/ml. Event-free survival was lower in patients with NGAL above this cutoff. Variables independently related to events were NGAL (HR 1.0035, 95% CI 1.0019–1.0052; p<0.0001) and male gender (HR 5.9, 95% CI 1.22–28.6; p=0.028).
ConclusionNGAL correlated with other biomarkers of renal function but not with BNP and was independently associated with outcomes.
A lipocalina associada a gelatinase de neutrófilos (NGAL) é um marcador precoce de injúria renal. O objectivo desse estudo foi avaliar o valor prognóstico desse biomarcador em pacientes com insuficiência cardíaca (IC) crônica estável.
MétodosIncluídos 61 pacientes com IC por disfunção sistólica sob tratamento otimizado por no mínimo seis meses. Os biomarcadores foram dosados basalmente e incluíam NGAL plasmático, microalbuminúria, creatinina sérica e o peptídeo natriurético do tipo B (BNP). A taxa de filtração glomerular estimada (eTFG) também foi calculada. O seguimento médio foi de 10,6±6,6 meses. O desfecho primário foi o tempo até o primeiro evento, definido como uma combinação de morte cardiovascular, hospitalização por IC ou visita à sala de emergência por IC. A análise multivariada foi feita pelo Modelo de Riscos Proporcionais de Cox.
ResultadosQuinze (24,6%) pacientes apresentaram um desfecho. Pacientes com desfecho apresentavam pior função renal e maiores níveis de NGAL (mediana 316 [variação interquartil de 122-705] versus 107 [78-170]; p=0,006). NGAL correlacionou-se significativamente com a creatinina (r=0,50, p<0,0001), albuminúria (r=0,33; p=0,008), e eTFG (r=−0,47; p=0,0001) mas não com BNP (r=0,003; p=0,97). O melhor corte de NGAL pela curva ROC foi 179 ng/mL. A sobrevida livre de eventos foi menor em pacientes com valores acima desse corte. As variáveis relacionadas de modo independente com eventos foram NGAL (razão de chances 1,0035, IC95% 1,0019-1,0052, p<0,0001) e sexo masculino (razão de chances 5,9, IC95% 1,22-28,6, p=0,028).
ConclusãoNGAL correlacionou-se positivamente com marcadores tradicionais de função renal, mas não com o BNP e foi preditor independente de eventos.
Heart failure (HF) is a severe disorder associated with high morbidity and mortality.1 Renal dysfunction as assessed by creatinine or estimated glomerular filtration rate (eGFR) has been associated with worse outcomes2–4 in patients with HF. However, creatinine is a late marker of renal function and early markers of the disorder are required.
Neutrophil gelatinase-associated lipocalin (NGAL) is an early marker of kidney injury and has been found to be elevated in both plasma and urine in patients with HF.5–7 NGAL is a 25 kDa protein covalently bound to matrix metalloproteinase-9 that was first isolated from neutrophils. It is produced by a wide variety of cells including respiratory and intestinal epithelial cells,8–10 endothelial cells, renal tubular cells and cardiomyocytes.7,11 Due to its association with kidney injury, inflammation and matrix remodeling, NGAL has been proposed as a marker of prognosis in patients with HF. However, data on patients under optimal treatment for chronic HF due to multiple etiologies are scarce. Additionally, the ability of NGAL to predict a decrease in eGFR over time has not been assessed. The aim of this study was to assess the prognostic role of plasma NGAL in patients with chronic HF and its relationship with traditional biomarkers of renal function and also with B-type natriuretic peptide (BNP), a marker of myocardial wall stress.
MethodsPatientsFrom April 2010 through July 2013, 61 patients with chronic HF, New York Heart Association (NYHA) functional class I–III, were prospectively included. Patients were recruited from the heart failure clinic of our medical school hospital. The diagnosis of HF was made on the basis of medical history, ongoing symptoms, and physical examination. All patients were on optimal medical treatment for HF for at least six months and had been clinically stable for at least three months before the study. All of them were on stable doses of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), beta-blockers, and spironolactone unless contraindicated. In all patients left ventricular ejection fraction (LVEF) was assessed by echocardiography using the Simpson method. All individuals had LVEF ≤50% at the time of inclusion. Patients under dialysis were excluded. The study was approved by the ethics committee of our hospital and written informed consent was obtained from all patients.
Study designThis was a prospective cohort study. Clinical characteristics and biomarkers were measured at baseline and were related to events in follow-up. Patients were followed at our HF clinic, with visits every three months. Mean follow-up was 10.6±6.6 months (maximum 2.05 years). The primary endpoint of the study was the time to first event, defined as a composite outcome of death from cardiovascular cause, admission for HF or emergency department visit for HF requiring intravenous diuretics. All endpoints were independently adjudicated. Creatinine was measured again at six months of follow-up to estimate eGFR at this point. The secondary endpoint was a 20% decrease in eGFR at six months.
Assessment of neutrophil gelatinase-associated lipocalinPlasma NGAL was assessed by the Triage NGAL Test (Alere Inc, San Diego, CA, USA), an immunoassay in a single-use plastic cartridge that contains a fluorescently labeled monoclonal antibody against NGAL labeled with a fluorescent dye and NGAL. There are built-in control features, including control immunoassays, to ensure that the test performs properly and that the reagents are functionally active. The test procedure involves the addition of several drops of whole blood or plasma to the sample port on the test device. After addition of the sample, the cells are automatically separated from the plasma via a filter. The sample reacts with fluorescent antibody conjugates within the reaction chamber and flows down the device detection lane by capillary action. The fluorescent antibody conjugates are captured on discrete solid-phase zones resulting in binding immunoassays that are specific for NGAL or the control antigens.
Assessment of other biomarkersVenous blood samples were taken and analyzed at the local laboratory. The other biomarkers included serum creatinine, microalbuminuria, and BNP. Serum creatinine was measured using standard techniques. Serum creatinine, age, body surface area, gender, and ethnicity were used to calculate eGFR using the Cockcroft-Gault method. First morning spot urine samples were taken with the patient in the upright position and transported immediately to the laboratory. Urinary albumin concentration (UAC) was determined using a turbidimetric assay and microalbuminuria was defined as UAC 25–200 mg/l, according to the manufacturer's indications. BNP testing was also performed on the Triage platform using a standard commercially available assay (Triage Assay, Alere Inc.).
Statistical analysisValues are expressed as mean values ± SD or absolute number and percentage. Differences between groups were investigated using the unpaired t test for independent samples or the chi-square test, as appropriate. Variables without a Gaussian distribution are expressed as median and interquartile range and were analyzed using the Mann-Whitney test. Receiver operating characteristic (ROC) curve analysis was used to determine the best NGAL cutoff to predict events. Kaplan-Meier event-free survival curves were constructed and compared using the log-rank test. Cox proportional hazards models were used to investigate the prospective association between NGAL and events during follow-up. Independent variables included in the model were age, gender, LVEF, creatinine, eGFR, albuminuria, BNP, and NGAL.
ResultsThe mean age of the study population was 61.6±11 years and 33 (54%) were male. Forty-seven (77%) patients were in NYHA functional class I or II, 14 (23%) in class III. Etiologies of HF were ischemic in 12 (19.7%), hypertensive in 32 (52.4%), idiopathic dilated cardiomyopathy (CM) in 10 (16.4%), alcoholic CM in 2 (3.3%), myocarditis in two (3.3%), peripartum CM in two (3.3%), and chemotherapy-induced CM in one (1.6%). Median NGAL in the whole cohort was 119 ng/ml (interquartile range 78.2–315). Baseline characteristics are shown in Table 1. Figure 1 depicts the correlation of NGAL with other biomarkers. As can be seen, there was a positive correlation with creatinine and albuminuria and a negative correlation with eGFR, but no correlation was observed between NGAL and BNP.
Baseline characteristics of the study population.
| Age (years) | 61.6±11.0 |
| Male | 33 (54%) |
| Hypertension | 32 (52.4%) |
| Diabetes | 20 (32.7%) |
| Previous MI | 11 (18.0%) |
| NYHA class III | 14 (23.0%) |
| Atrial fibrillation | 10 (16.4%) |
| LBBB | 5 (8.2%) |
| LVEF (%) | 37.7±8.2 |
| Hemoglobin (mg/dl) | 13±1.5 |
| Creatinine (mg/dl) | 1.32±0.7 |
| eGFR (ml/min) | 54.4±20 |
| Albuminuria (mg/l)a | 12 (3.8–37.4) |
| BNP (pg/ml)a | 61 (23.4–131.5) |
| NGAL (ng/ml)a | 119 (78.2–315) |
| Beta-blockers | 59 (96.7%) |
| ACE inhibitors/ARBs | 60 (98.3%) |
| Spironolactone | 45 (73.7%) |
ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blockers; BNP: B-type natriuretic peptide; eGFR: estimated glomerular filtration rate; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NGAL: neutrophil gelatinase-associated lipocalin; NYHA: New York Heart Association.
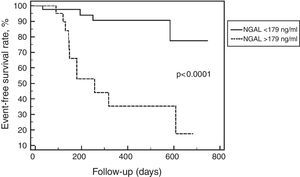
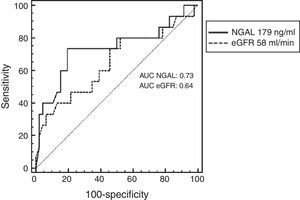
Fifteen (24.6%) patients reached the primary endpoint. Baseline NGAL was higher in these patients, as depicted in Figure 2. Patients with events were also more likely to have worse renal function at baseline as assessed by creatinine, eGFR and microalbuminuria. Univariate comparison of patients with and without events is shown in Table 2. The best NGAL cutoff as determined by ROC curve analysis was 179 ng/ml, with sensitivity of 73.3%, specificity of 80.4% and area under the curve (AUC) of 0.73 (p=0.007). Event-free survival was lower in patients with NGAL above this cutoff as shown in Figure 3. Figure 4 depicts ROC curves of NGAL and eGFR in the prediction of the primary endpoint. NGAL added to eGFR, with a 14% increase in the AUC. Using Cox proportional hazards models, only NGAL (hazard ratio [HR] 1.0035, 95% confidence interval [CI] 1.0019–1.0052, p<0.0001) and male gender (HR 5.9, 95% CI 1.22–28.6, p=0.028) were independently related to time to first event.
Comparison between patients with and without events.
| Characteristic | With events (n=15) | Without events (n=46) | p |
|---|---|---|---|
| Age (years) | 65.8±11.7 | 60.3±10.7 | 0.09 |
| Male | 12 (80%) | 21 (45.6%) | 0.04 |
| Hypertension | 11 (73.3%) | 34 (74%) | 0.75 |
| Diabetes | 6 (40%) | 14 (30.4%) | 0.56 |
| Previous MI | 4 (26.7%) | 7 (15.2%) | 0.53 |
| NYHA class III | 5 (33.3%) | 9 (19.6%) | 0.45 |
| Atrial fibrillation | 4 (26.6%) | 6 (13%) | 0.40 |
| Left bundle branch block | 2 (13.3%) | 3 (6.5%) | 0.76 |
| LV ejection fraction (%) | 39.6±6.4 | 37±8.7 | 0.31 |
| Hemoglobin (mg/dl) | 12.9±1.4 | 13.1±1.5 | 0.49 |
| Creatinine (mg/dl) | 1.6±0.9 | 1.2±0.6 | 0.03 |
| eGFR (ml/min) | 46±23.3 | 57±18.3 | 0.06 |
| Albuminuria (mg/l)a | 40 (17–77.7) | 7.5 (3.8–28) | 0.02 |
| BNP (pg/ml)a | 62.7 (32–176) | 59.9 (16.7–130) | 0.52 |
| NGAL (ng/ml)a | 316 (122–705) | 107 (78–170) | 0.006 |
BNP: B-type natriuretic peptide; eGFR: estimated glomerular filtration rate; LV: left ventricle; MI: myocardial infarction; NGAL: neutrophil gelatinase-associated lipocalin; NYHA: New York Heart Association.
Receiver operating characteristic curve analysis of neutrophil gelatinase-associated lipocalin and estimated glomerular filtration rate for predicting cardiovascular mortality, heart failure hospitalization or emergency department visit for heart failure. AUC: area under the curve; eGFR: estimated glomerular filtration rate; NGAL: neutrophil gelatinase-associated lipocalin.
In the present study, we found that plasma NGAL is a powerful predictor of long-term outcomes in patients with chronic HF under optimal medical treatment who were managed by HF specialists. NGAL was a strong predictor of clinical outcomes such as cardiovascular death and HF hospitalizations. It was in fact stronger than BNP, which is considered to be the standard HF biomarker, and performed better than conventional measures of renal function such as creatinine, eGFR, and microalbuminuria. Importantly, our data indicate that even in patients without chronic kidney disease or albuminuria, increased NGAL is associated with unfavorable outcomes.
In contrast with creatinine, NGAL is a tubular damage marker that has been found to be elevated early after acute kidney injury (AKI). Dent et al. demonstrated that NGAL was effective in identifying AKI 2–3 hours after cardiopulmonary bypass surgery as compared with 2–3 days for creatinine.12 In patients admitted for HF, Damman et al. demonstrated that admission serum NGAL levels were associated with increased risk of subsequent worsening of renal function.5 Additionally, in patients with acutely decompensated HF, Maisel et al. found that serum NGAL measured at discharge was a better risk predictor of 30-day events than BNP, creatinine or eGFR.13
In patients with chronic HF, few such studies have been carried out. In the GISSI-HF study, urinary NGAL was measured along with other urinary markers of tubular damage. All of them were associated with a poor clinical outcome, even when eGFR was normal.6 In the CORONA study, the ability of plasma NGAL to predict clinical events was assessed in patients with chronic HF.14 Multivariate analysis revealed that NGAL added significant information when adjusting for clinical variables, but was no longer significant after further adjustment for apolipoprotein A-1, C-reactive protein (CRP), eGFR, and N-terminal pro-brain natriuretic peptide (NT-proBNP). However, there are some important differences between the CORONA study and the present study. The former included patients with ischemic systolic HF, while patients with multiple etiologies were included in our study. Besides, the CORONA study included elderly patients.
Interestingly, NGAL seems to predict cardiovascular outcomes in the community. Plasma NGAL was measured in 1393 Rancho Bernardo Study participants without cardiovascular disease (CVD), who were followed for a mean of 11 years. NGAL was a significant predictor of mortality and CVD in this elderly cohort, independently of traditional risk factors and kidney function and in addition to NT-proBNP and CRP.15
Taken together, these results indicate that plasma NGAL provides important prognostic information and is not merely serving as a surrogate measure of renal function. It has been related to inflammation16 and matrix remodeling17 and is expressed at high levels in the heart and in atherosclerotic plaques.18 There are important differences between plasma and urinary NGAL. NGAL mRNA is markedly up-regulated within the distal nephron during ischemic or toxic renal injury, and the secretion of NGAL into the urine seems to comprise the major fraction of urinary NGAL. By contrast, although plasma NGAL levels increase during acute and chronic renal impairment, the kidney seems not to be the only source of circulating NGAL. Kidney injury and other ischemic and inflammatory processes cause up-regulation of NGAL production in other tissues and cells, which probably represents the main source of plasma NGAL.19,20 Whether such differences may result in significant differences in plasma NGAL performance as compared with urinary NGAL is not known. To date, no head-to-head comparison between these two biomarkers has been carried out.
In the present study NGAL correlated with creatinine, eGFR, and albuminuria but not with BNP. In addition, BNP, a standard HF biomarker, was not associated with outcomes. A possible explanation for this is the fact that all patients were on optimal medical treatment for HF for at least six months. BNP has been shown to respond to HF treatment, and some studies in fact report benefits in using natriuretic peptides to guide treatment.21,22 Median BNP in our population was quite low (61 pg/ml), reflecting excellent medical treatment. These results suggest that in chronic HF patients under optimal treatment, BNP is no longer a good risk marker since it has been reduced in response to treatment. In this scenario markers of renal injury and inflammation such as NGAL seem to be superior to wall stress biomarkers.
Two limitations of the present study must be addressed. This is a small, single-center study with a small number of events, which may limit statistical analysis. Therefore, our data need to be validated in larger prospective studies. Secondly, we studied a heterogeneous population, with multiple etiologies for HF. Since this is a small sample, we could not control for issues related to specific etiologies. Despite these limitations, this was a prospective longitudinal study with valuable information regarding prognosis in stable HF patients, using a simple biomarker that can be easily used in daily clinical practice. Studies in a large population addressing the natural course of NGAL in chronic HF and its potential as a treatment target are warranted.
ConclusionIn this study we found that in patients with chronic HF under optimal medical treatment, NGAL correlates with traditional markers of renal function such as creatinine, eGFR, and albuminuria but not with BNP. NGAL was associated with outcomes, independently of clinical variables, conventional measures of renal function, and BNP.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.