To analyze long-term survival and predictors of mortality in patients evaluated for transcatheter aortic valve implantation (TAVI) depending on the decision taken by the heart team.
MethodsAll patients with severe aortic stenosis and high surgical risk evaluated for TAVI between June 2008 and June 2012 were included. Patients were grouped according to the therapeutic strategy decided by the heart team. Mean follow-up was 16.6 months (maximum 55.3).
ResultsA total of 149 patients were evaluated: 79 were accepted for TAVI, 12 had no current indication for valve replacement and were deferred, 13 were redirected to conventional surgery and 45 received medical treatment. The evaluated patients had a mean age of 83.7 years and a mean EuroSCORE of 19.8±12.3. Median survival free from all-cause death was 34.7 months (95% CI 27.1–42.3) in the TAVI group, 47.4 months (95% CI 0–97.4) in the deferred intervention group, not available in the surgery group and 8.2 months (95% CI 5.6–10.9) in the medical treatment group (log-rank p<0.001). After multivariable adjustment, only treatment group remained as an independent predictor of mortality. Considering the TAVI group as the reference category, the adjusted hazard ratio for all-cause death was 0.70 (95% CI 0.24–2.04) for the deferred intervention group, 0.16 (95% CI 0.02–1.19) for the surgery group and 2.47 (95% CI 1.46–4.18) for the medical treatment group.
ConclusionThe decision taken by the heart team on potential candidates for TAVI has a decisive prognostic significance, as those who are unsuitable for any kind of valve replacement have a significantly higher mortality.
Analisar a sobrevivência a longo prazo e os fatores preditores de mortalidade de doentes avaliados para implantação percutânea da válvula aórtica (TAVI), dependendo da decisão tomada pela Heart Team.
MétodosForam incluídos todos os doentes com estenose aórtica grave e elevado risco cirúrgico avaliados para TAVI desde junho 2008 a junho 2012. Os doentes foram agrupados de acordo com a estratégia terapêutica decidida pela Heart Team. Seguimento médio 16,6 meses (máximo 55,3).
ResultadosFoi avaliado um total de 149 doentes, dos quais 79 foram aceites para TAVI, 12 não apresentaram indicação para substituição valvular e foram diferidos, 13 foram redirecionados para cirurgia convencional e 45 receberam tratamento médico. Os doentes avaliados tinham uma idade média de 83,7 anos e um EuroSCORE médio de 19,8±12,3. A sobrevida média livre de todas as causas de morte foi 34,7 meses (IC 95% 27,1-42,3) no grupo TAVI, de 47,4 meses (IC 95% 0-97,4) no grupo de intervenção diferido, não disponível no grupo de cirurgia e de 8,2 meses (IC 95% 5,6-10,9) no grupo de tratamento médico (Log rank p <0,001). Após o ajuste de múltiplas variáveis, só o grupo de tratamento permaneceu como um fator preditor independente da mortalidade. Considerando o grupo TAVI como categoria de referência, a taxa de risco ajustada para todas as causas de morte foi de 0,70 (IC 95% 0,24-2,04) para o grupo de intervenção diferido, de 0,16 (IC 95% 0,02-1,19) para o grupo da cirurgia e de 2,47 (IC 95% 1,46-4,18) para o grupo de tratamento médico.
ConclusãoA decisão tomada pela Heart Team relativamente aos potenciais candidatos para TAVI tem um impacto decisivo no prognóstico dado que os que não são elegíveis para qualquer tipo de substituição valvular apresentam uma mortalidade significativamente maior.
Aortic stenosis (AS) is the most prevalent valvulopathy in developed countries. The condition is mainly of degenerative etiology1 and is associated with aging, and is therefore becoming more frequent as the average age of the population increases.2 It is a chronic and progressive disease characterized by lipid accumulation, inflammation and calcification, with many similarities in terms of pathophysiology and risk factors to atherosclerosis.3 Once the patient has developed symptoms, the prognosis of unoperated patients is very poor, with a two-year survival around 21%, so they should be referred quickly for surgical aortic valve replacement (SAVR).4–6 However, many of them are considered at high risk for surgery due to advanced age and comorbidities.7,8
Transcatheter aortic valve implantation (TAVI) has emerged in recent years as a less invasive alternative treatment for high-risk patients.9 This strategy has been shown to reduce mortality and hospitalizations compared to conservative management in patients considered unfit for surgery, and survival rates are similar compared to surgical valve replacement in patients at high surgical risk but still considered operable.10,11 Nevertheless some candidates for TAVI are denied this technique for various reasons.
It is known that the mortality of patients who are not operated is high and increases with age, left ventricular dysfunction, heart failure and kidney failure.12 However, there are few data on patients treated conservatively after the recent emergence of this percutaneous intervention.13,14 The objective of this study is to analyze the survival and predictors of mortality of patients with AS and high surgical risk included in a TAVI evaluation program depending on the final treatment strategy decided by the heart team.
MethodsPatientsIn accordance with standard practice, all patients with AS and indication for valve replacement (severe AS and related symptoms) were evaluated by a multidisciplinary team, including clinical and interventional cardiologists, cardiac surgeons and anesthesiologists (the heart team). Further visits by heart team members were made when needed, during which direct patient examination and a qualitative assessment of frailty were performed. Patients at high risk for conventional surgery were considered for TAVI, usually because of age, comorbidities, previous cardiac surgery, ventricular dysfunction or concomitant coronary artery disease, as were patients who refused SAVR. TAVI candidates underwent the standard protocol, according to the device manufacturer's recommendations, to confirm that they were suitable for this technique.15,16 All patients referred for TAVI evaluation between June 2008 and June 2012 were prospectively included in the study.
Evaluation and treatment allocationThe first step in the evaluation was to confirm that there was indication for valve replacement according to the guidelines of the European Society of Cardiology.17 Data from transthoracic echocardiographic study were reviewed to determine the severity of AS. The echocardiographic criteria of severity were those established by clinical practice guidelines: aortic valve area <1 cm2, mean gradient >40 mmHg and maximum jet velocity >4 m/s. Also, the presence of symptoms (angina, syncope and exertional dyspnea) secondary to valvular heart disease was determined by reviewing medical records and clinical visits.
Subsequently, surgical risk with regard to age, cardiovascular risk factors, comorbidities, logistic EuroSCORE calculation18 and baseline patient characteristics was evaluated, and the presence of contraindications for the intervention were ruled out: acute myocardial infarction in the last 30 days, hemodynamic instability, severe left ventricular dysfunction with ejection fraction (LVEF) less than 20% or life expectancy of less than 12 months because of non-cardiac problems.
Patients who passed the initial assessment also underwent transesophageal echocardiography, cardiac catheterization and multidetector computed tomography (MDCT). The objective of the transesophageal study was to confirm the severity, mechanism and hemodynamics of AS, to measure the dimensions and morphology of the aortic valve annulus and to evaluate the existence of aortic insufficiency or other associated valvulopathies. Patients underwent coronary angiography to detect the existence of significant atherosclerotic lesions, and lower limb arterial angiography to confirm the criteria to perform the procedure by a transfemoral approach. Finally, MDCT was also used to study the left ventricular outflow tract and the aortic annulus and to measure the caliber of the iliac and femoral arteries.
Finally, the results were presented to the heart team to make a final decision. Those who were considered suitable for the procedure underwent TAVI. Some were considered not to have indication for valve replacement at that time because of non-severe or only mildly symptomatic AS, and the heart team decided to defer invasive treatment. After the study protocol for TAVI candidates, some were considered anatomically unsuitable for TAVI or had other significant concomitant valve disease, without excessive surgical risk, so conventional surgery was reconsidered by the heart team and they underwent surgical valve replacement. The remaining patients were those who did not meet the appropriate anatomic conditions for a percutaneous technique, those with excessive risk and those who refused any intervention, and these received medical treatment only.
ProcedureThe TAVI procedure was performed according to standard techniques described previously.10 In all cases the implanted valve was the Edwards SAPIEN Transcatheter Heart Valve (Edwards Lifesciences). Initially the approach was exclusively transfemoral with surgical exposure of the femoral artery by vascular surgery. However, from February 2011, a transapical TAVI program was introduced, performed by cardiac surgeons.19 The approach was selected depending on the size of the annulus and femoral arteries: in patients with an annulus between 18 and 25 mm and femoral artery diameter >7 mm a transfemoral approach was used, while in patients with an aortic annulus between 25 and 27 mm or between 18 and 25 mm with femoral artery diameter <7 mm, transapical access was chosen once it became available. The cutoff point of 7 mm was reduced to 6 mm after the development of the new generation of valve and transfemoral delivery catheters in 2010 (XT valve with the NovaFlex delivery system).20 Patients with annular diameters outside these limits or inadequate transfemoral access before the introduction of transapical access were considered unsuitable for the percutaneous technique.
Data collectionInformation on baseline characteristics, cardiovascular risk factors, comorbidities, echocardiographic data and symptoms was collected in a specially designed prospective database. Follow-up information in the different groups was obtained prospectively by medical consultations, telephone interviews and review of computerized medical records. The day the heart team took the final decision on the most appropriate treatment strategy for each patient was considered as day 0 of the follow-up.
Definition of variablesThe primary endpoint of the study was all-cause mortality during follow-up. Additionally, the cause of death was classified as cardiovascular or non-cardiovascular according to the Valve Academic Research Consortium criteria recommended for TAVI studies.21 The secondary endpoint was event-free survival from the composite endpoint of major adverse cardiovascular events (MACE) defined as death, hospitalization for heart failure, non-fatal myocardial infarction or non-fatal stroke.
Statistical analysisContinuous variables are presented as mean ± standard deviation and categorical variables are presented as relative frequencies (percentages). Statistical analysis of the results was performed by intention to treat, allocating the patient to the treatment group decided by the heart team. The analysis of all-cause death and MACE-free survival was performed by the Kaplan–Meier method and groups were compared with the log-rank test. Analysis of prognostic factors was performed with the Cox proportional hazards model, univariate and multivariate, calculating the hazard ratios for all-cause death. p values and 95% confidence intervals (CI) are bilateral, with a cutoff of 0.05 for statistical significance. All analyses were performed using SPSS® Statistics software, version 20 (IBM®).
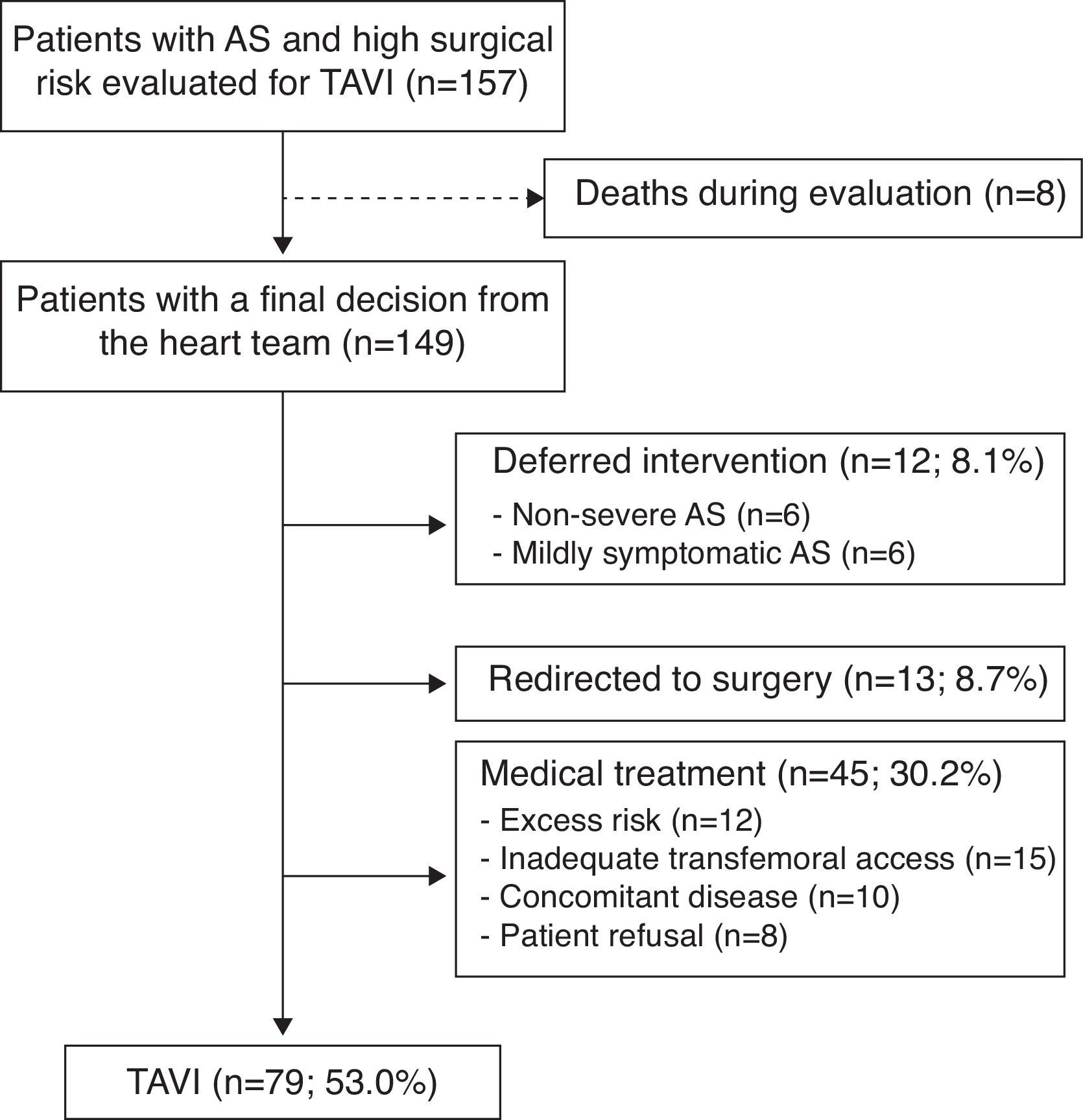
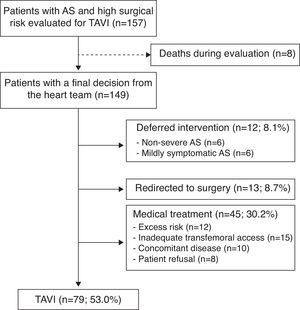
ResultsBetween June 2008 and June 2012 a total of 157 patients were evaluated for a possible TAVI procedure. The heart team discussed each case and decided the most appropriate treatment strategy. The mean time between the initial presentation of the case and the final decision of the heart team was 0.7 months (minimum 0.1; maximum 12.3). Of the 157 cases included in the evaluation protocol, eight patients died during the initial assessment before a final therapeutic decision of the heart team could be taken, and were excluded from the study. Of the 149 patients with a final decision from the heart team 79 (53.0%) were accepted for TAVI, 12 (8.1%) had the valve replacement deferred because of non-severe or only mildly symptomatic AS, 13 (8.7%) were redirected to conventional surgery and 45 (30.2%) received medical treatment for different reasons: excess risk, unfavorable iliac or femoral arterial anatomy (before the introduction of transapical access), concomitant disease (any short-term life-threatening non-cardiac condition) or patient refusal (Figure 1). Mean follow-up was 16.6±14.1 months (maximum 55.3). No patients were lost during follow-up. All were monitored, at least by telephone interview.
All patients in the TAVI and surgery groups underwent the procedure to which they were allocated. Of the patients undergoing TAVI, 64 (81.0%) were treated by transfemoral access and 15 (19.0%) by transapical access. Implant success was achieved in 75 of the 79 TAVI patients (94.9%). During follow-up, one patient in the deferred intervention group and three of the medical treatment group underwent SAVR. None of the patients undergoing TAVI underwent surgery during follow-up. In the medical treatment group two patients received percutaneous aortic valvuloplasty.
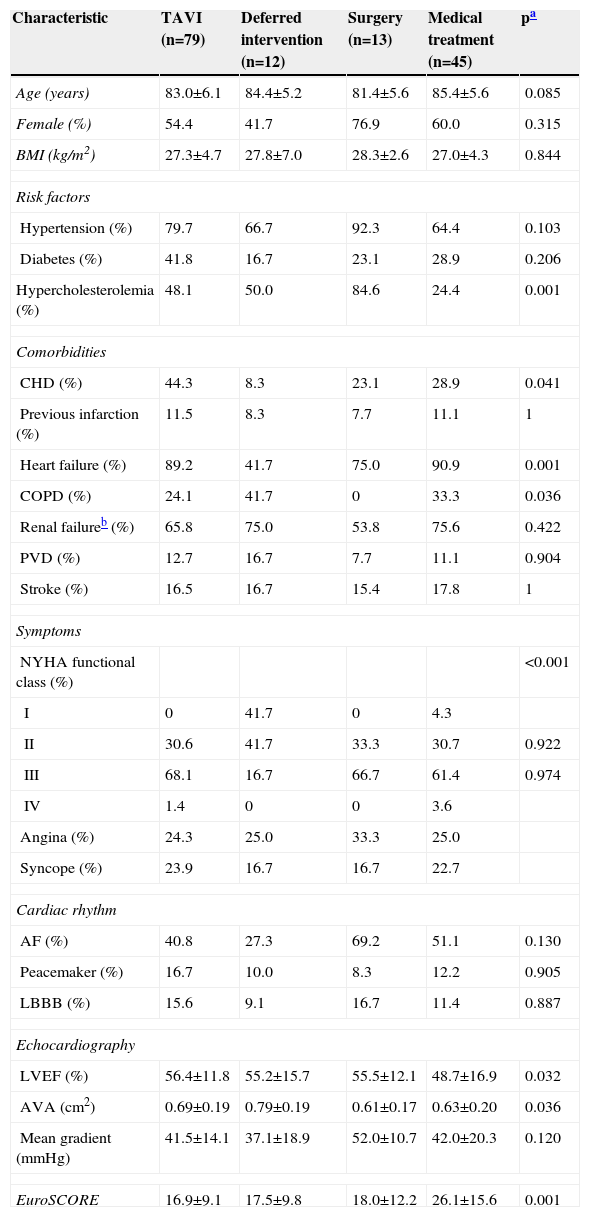
Patients evaluated for TAVI had a mean age of 83.7 years and 57.0% were women. Mean EuroSCORE was 19.8±12.3. Patients in the medical treatment group had older age, more comorbidities and higher surgical risk, as shown by lower LVEF and higher EuroSCORE. Baseline characteristics and echocardiographic data in the different treatment groups are shown in Table 1.
Baseline characteristics of the patients and echocardiographic findings.
| Characteristic | TAVI (n=79) | Deferred intervention (n=12) | Surgery (n=13) | Medical treatment (n=45) | pa |
|---|---|---|---|---|---|
| Age (years) | 83.0±6.1 | 84.4±5.2 | 81.4±5.6 | 85.4±5.6 | 0.085 |
| Female (%) | 54.4 | 41.7 | 76.9 | 60.0 | 0.315 |
| BMI (kg/m2) | 27.3±4.7 | 27.8±7.0 | 28.3±2.6 | 27.0±4.3 | 0.844 |
| Risk factors | |||||
| Hypertension (%) | 79.7 | 66.7 | 92.3 | 64.4 | 0.103 |
| Diabetes (%) | 41.8 | 16.7 | 23.1 | 28.9 | 0.206 |
| Hypercholesterolemia (%) | 48.1 | 50.0 | 84.6 | 24.4 | 0.001 |
| Comorbidities | |||||
| CHD (%) | 44.3 | 8.3 | 23.1 | 28.9 | 0.041 |
| Previous infarction (%) | 11.5 | 8.3 | 7.7 | 11.1 | 1 |
| Heart failure (%) | 89.2 | 41.7 | 75.0 | 90.9 | 0.001 |
| COPD (%) | 24.1 | 41.7 | 0 | 33.3 | 0.036 |
| Renal failureb (%) | 65.8 | 75.0 | 53.8 | 75.6 | 0.422 |
| PVD (%) | 12.7 | 16.7 | 7.7 | 11.1 | 0.904 |
| Stroke (%) | 16.5 | 16.7 | 15.4 | 17.8 | 1 |
| Symptoms | |||||
| NYHA functional class (%) | <0.001 | ||||
| I | 0 | 41.7 | 0 | 4.3 | |
| II | 30.6 | 41.7 | 33.3 | 30.7 | 0.922 |
| III | 68.1 | 16.7 | 66.7 | 61.4 | 0.974 |
| IV | 1.4 | 0 | 0 | 3.6 | |
| Angina (%) | 24.3 | 25.0 | 33.3 | 25.0 | |
| Syncope (%) | 23.9 | 16.7 | 16.7 | 22.7 | |
| Cardiac rhythm | |||||
| AF (%) | 40.8 | 27.3 | 69.2 | 51.1 | 0.130 |
| Peacemaker (%) | 16.7 | 10.0 | 8.3 | 12.2 | 0.905 |
| LBBB (%) | 15.6 | 9.1 | 16.7 | 11.4 | 0.887 |
| Echocardiography | |||||
| LVEF (%) | 56.4±11.8 | 55.2±15.7 | 55.5±12.1 | 48.7±16.9 | 0.032 |
| AVA (cm2) | 0.69±0.19 | 0.79±0.19 | 0.61±0.17 | 0.63±0.20 | 0.036 |
| Mean gradient (mmHg) | 41.5±14.1 | 37.1±18.9 | 52.0±10.7 | 42.0±20.3 | 0.120 |
| EuroSCORE | 16.9±9.1 | 17.5±9.8 | 18.0±12.2 | 26.1±15.6 | 0.001 |
AF: atrial fibrillation; AVA: aortic valve area; BMI: body mass index; CHD: coronary heart disease; COPD: chronic obstructive pulmonary disease; LBBB: left bundle bunch block; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PVD: peripheral vascular disease; TAVI: transcatheter aortic valve implantation.
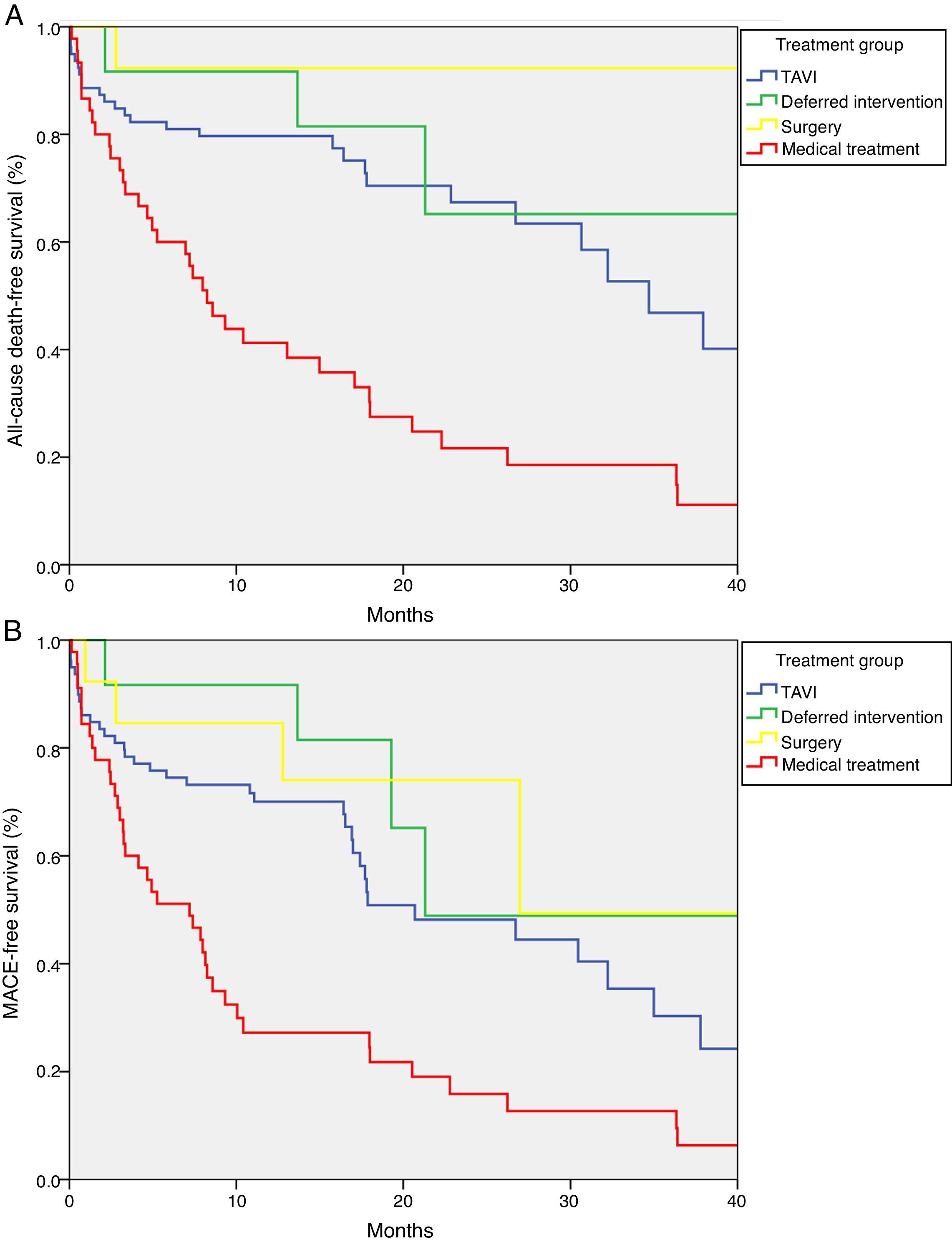
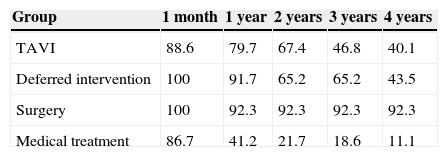
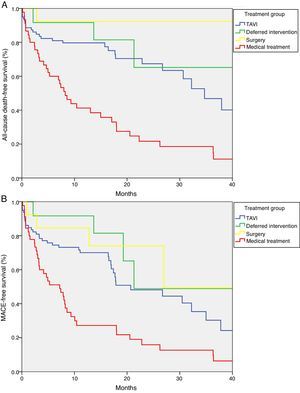
Median survival free from all-cause death was 34.7 months (95% CI 27.1–42.3) in the TAVI group, 47.4 months (95% CI 0–97.4) in the deferred intervention group, not available in the surgery group because more than 50% survived to the end of follow-up (mean survival 51.3 months, 95% CI 43.7–58.9), and 8.2 months (95% CI 5.6–10.9) in the medical treatment group (log-rank p<0.001) (Figure 2A). Overall survival at one year was 79.7% in the TAVI group, 91.7% in the deferred intervention group, 92.3% in the surgery group and 41.2% in the medical treatment group. Overall survival at two years was 67.4% in the TAVI group, 65.2% in the deferred intervention group, 92.3% in the surgery group and 21.7% in the medical treatment group (Table 2). The cause of death was cardiovascular in 50.0% of deaths in the TAVI group, 100% of deaths in the deferred intervention and surgery groups and 83.3% of deaths in the medical treatment group.
Survival at 1 month, 1 year and 2 years by treatment group (%).
| Group | 1 month | 1 year | 2 years | 3 years | 4 years |
|---|---|---|---|---|---|
| TAVI | 88.6 | 79.7 | 67.4 | 46.8 | 40.1 |
| Deferred intervention | 100 | 91.7 | 65.2 | 65.2 | 43.5 |
| Surgery | 100 | 92.3 | 92.3 | 92.3 | 92.3 |
| Medical treatment | 86.7 | 41.2 | 21.7 | 18.6 | 11.1 |
TAVI: transcatheter aortic valve implantation.
Median event-free survival for the MACE composite secondary endpoint was 20.7 months (95% CI 9.6–31.8) in the TAVI group, 21.3 months (CI not calculable) in the deferred intervention group, 27.0 months (CI not calculable) in the surgery group and 7.2 months (95% CI 3.0–11.4) in the medical treatment group (log-rank p<0.001) (Figure 2B).
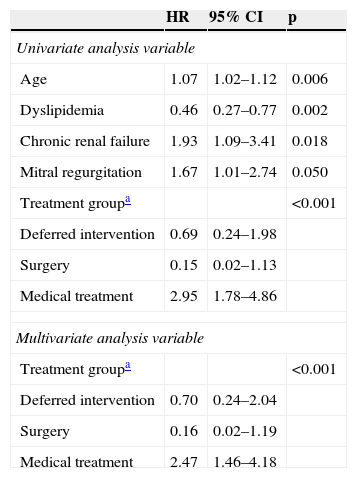
Predictors of mortalityIn univariate analysis, the following variables: age, dyslipidemia, chronic renal failure, significant mitral regurgitation (grade 3 or 4) and treatment group, showed a statistically significant association with mortality during follow-up. All significant variables in univariate analysis were entered into the multivariate Cox proportional hazards model, in which only treatment group remained as an independent predictor of mortality (Table 3). Compared to the TAVI group, used as the reference category, the adjusted hazard ratio for all-cause death was 0.70 (95% CI 0.24–2.04) for the deferred intervention group, 0.16 (95% CI 0.02–1.19) for the conventional surgery group and 2.47 (95% CI 1.46–4.18) for the medical treatment group.
Predictors of mortality by the Cox proportional hazards model.
| HR | 95% CI | p | |
|---|---|---|---|
| Univariate analysis variable | |||
| Age | 1.07 | 1.02–1.12 | 0.006 |
| Dyslipidemia | 0.46 | 0.27–0.77 | 0.002 |
| Chronic renal failure | 1.93 | 1.09–3.41 | 0.018 |
| Mitral regurgitation | 1.67 | 1.01–2.74 | 0.050 |
| Treatment groupa | <0.001 | ||
| Deferred intervention | 0.69 | 0.24–1.98 | |
| Surgery | 0.15 | 0.02–1.13 | |
| Medical treatment | 2.95 | 1.78–4.86 | |
| Multivariate analysis variable | |||
| Treatment groupa | <0.001 | ||
| Deferred intervention | 0.70 | 0.24–2.04 | |
| Surgery | 0.16 | 0.02–1.19 | |
| Medical treatment | 2.47 | 1.46–4.18 | |
CI: confidence interval; HR: hazard ratio; TAVI: transcatheter aortic valve implantation.
This single-center observational study of unselected patients shows that the prognosis of patients with severe AS and high surgical risk evaluated for TAVI is influenced by the final decision of the heart team. This prognosis is more favorable for patients who undergo aortic valve replacement, by either a percutaneous or a surgical approach, and for those who do not have a definite indication for valve replacement at the time of evaluation (deferred group). Conversely, it is poor for those considered unsuitable for TAVI or SAVR (medical treatment group), either because they have a contraindication for the percutaneous technique or because they refuse any invasive treatment. These patients have very high mortality, mostly from cardiovascular causes.
Role of transcatheter aortic valve implantationVarious studies have shown poor outcomes for patients with severe AS treated conservatively. The introduction of TAVI has transformed the prospects for these patients, who now have a less invasive alternative to surgery for the treatment of their valvular heart disease. The TAVI procedure has demonstrated a reduction in mortality and hospitalization and improvement in cardiac symptoms compared to medical therapy in these patients.10
The interest of this study lies not in analysis of the effectiveness of TAVI, for which there are several larger and multicenter registries, as well as a few randomized clinical trials, but in its description of the differences in the natural history of the disease according to treatment strategy. However, it is noteworthy that survival rates in our TAVI group were similar to data in the literature, with a one-year survival of 80% vs. 69–83% reported in various publications.10,11,13,22 It should be noted that ours is a national reference center with a volume of TAVI procedures slightly above the average in Portugal (19.8 annual procedures compared to the average of 16.4 per center recorded between 2010 and 2011).23
Treatment groupsThe medical treatment group had the most adverse prognosis, with a survival rate of 41% at one year and 22% at two years, similar to that described in the literature before the introduction of TAVI. In this patient group aortic balloon valvuloplasty has not shown good results in the medium to long term, due to a high incidence of restenosis.24,25 There is no pharmacological treatment with a prognostic benefit in AS, so medical management should be directed to symptomatic relief. Our results support this approach, especially when taking into account that life expectancy is usually very limited for those who are not candidates for TAVI. This does not mean that patients who were considered unsuitable after evaluation should have been accepted for TAVI, but rather that they represent a very high risk population, with a particularly unfavorable prognosis.
Regarding the deferred intervention and conventional surgery groups, although the Kaplan–Meier curves seem to indicate improved survival, there were no significant differences in survival compared to the TAVI group due to the limited number of patients in each group. The relatively low number of patients treated with SAVR in our series is explained by the fact that patients initially considered suitable for SAVR (low or intermediate risk) were not included in the TAVI evaluation program.
Predictors of mortalityOur results suggest that the final treatment strategy adopted by the heart team is the main predictor of mortality in patients evaluated for TAVI. This is mainly due to the higher event rate in the medical treatment group, with a hazard ratio for all-cause mortality close to 2.5 compared to the group considered suitable for TAVI. Although in the deferred intervention and surgery groups the hazard ratio was lower than 1, in both cases the confidence intervals were wide and included the value 1, so it cannot be concluded that the prognosis of these groups is better than the TAVI group.
Comparison with previous studiesThere are very few studies designed to evaluate the prognosis of patients with AS and high surgical risk depending on their suitability for TAVI. Wenaweser et al.13 studied the evolution of a cohort of patients with severe AS at high risk according to treatment strategy, comparing medical treatment, SAVR and TAVI. Similarly to our study, a multidisciplinary team taking into account the patient's decision assigned the final treatment. Mortality at 30 months was significantly higher in the medical treatment group (61.5%) compared to the surgery group (22.4%) and TAVI group (22.6%). In this study, the variables of medical treatment, advanced age (>80 years), peripheral vascular disease and atrial fibrillation were associated with mortality at 30 months in multivariate analysis.
Ben-Dor et al. analyzed the prognosis of patients not eligible for TAVI clinical trials because they did not meet inclusion/exclusion criteria.14 The main exclusion criteria were low Society of Thoracic Surgeons score (<10%), peripheral vascular or aortic disease, aortic valve area >0.8 cm2, significant coronary artery disease requiring revascularization, and severe renal insufficiency. The study population was divided into two groups, one unsuitable for surgery who received medical treatment with or without aortic valvuloplasty and the other who underwent SAVR. Mortality in the medical/valvuloplasty treatment group was significantly higher than in the surgery group, 39.6% vs. 22.9% at one year and 53.4% vs. 28.1% at two years, respectively. Multivariate analysis identified renal failure, New York Heart Association class IV and systolic blood pressure as independent predictors of mortality in the medical group. This paper studied a highly selected population, and so the treatment groups were not comparable with our study. Furthermore, unlike our study, they excluded patients with significant coronary artery disease requiring revascularization and those with severe renal insufficiency (defined as creatinine >3.0 mg/dl or hemodialysis).
In PARTNER cohort B (inoperable patients with severe symptomatic AS), mortality from any cause was 50.7% at one year and 68.0% at two years for the standard therapy group.10,26 Cardiovascular mortality was 44.6% and 62.4%, respectively. The main difference from our study was that medically treated patients were not considered unsuitable for TAVI by the heart team, but were assigned to standard therapy by the randomization process.
Relevance and limitations of the studyThe prognostic data derived from this study are important for providing the patient with detailed and accurate information about the multiple treatment options available in high surgical risk AS. Most patients and families face this decision with many doubts because of fear of a major intervention, uncertainty about the potential improvement and ignorance of the natural history of the disease, as well as other personal, cultural and religious factors. We must not forget that a considerable proportion of our population (6.7%) refused any invasive treatment because of personal decisions by patients and relatives. This decision, especially in such an elderly population, should of course be respected, but every effort should be made to give comprehensive information about their expected prognosis.
Despite the limitation of being a single-center study with a small number of patients, which reduces the power of the analysis, this was a prospective cohort study with a very long follow-up in all patients. Furthermore, the evaluation was performed in all patients by the same heart team, which gives the results greater consistency. Finally, the observational design of the study with no exclusion criteria more accurately represents daily clinical practice.
Another limitation of the study should be noted. Many patients continued their monitoring in the hospitals from which they were referred and follow-up was done through telephone interviews. Therefore, we could not obtain information about possible complications of intervention, such as aortic regurgitation or need for pacemaker implantation.
ConclusionsThe therapeutic decision taken by the heart team in patients with severe AS and high surgical risk, considered potential candidates for TAVI, is the main factor predicting long-term mortality. Those who are unsuitable for invasive treatment and receive medical treatment have a significantly higher mortality.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.