Frequent premature ventricular contractions (PVCs) originating from the right ventricular outflow tract (RVOT) are usually considered a benign entity and the ECG is typically normal. The aim of this study was to assess whether upward displacement of the ECG to the second intercostal space (ICS) would reveal any abnormal pattern.
MethodsA total of 18 consecutive patients with apparently normal hearts were studied, mean age 44±16 years, 12 women, who underwent catheter ablation of the RVOT due to frequent PVCs. A 12-lead ECG was performed in the standard position and repeated in a higher position, at the level of the second ICS. Three-dimensional bipolar electroanatomical voltage mapping (EVM) was performed in all patients and low voltage areas (LVAs) were defined as areas with amplitude <1.5 mV.
ResultsThe ECG in the second ICS was normal in eleven patients but in seven (39%) it revealed a pattern of ST-segment elevation in V1. EVM revealed the presence of LVAs in six patients (33%) which included the earliest activation site (EAS) in five. The ST elevation was associated with the presence of LVAs (p<0.0001) and with the LVAs at the EAS (p=0.002).
ConclusionIn this group of patients with apparently normal hearts and with frequent PVCs of the RVOT, upward displacement of the ECG revealed the presence of ST elevation in more than one third of patients, and the ST elevation was associated with the presence of LVAs in the RVOT.
A extrasistolia ventricular (ESV) frequente da camara de saída do VD (CSVD) é em geral uma patologia benigna e o ECG é tipicamente normal. O objectivo deste estudo é avaliar se o ECG efetuado no 2.° espaço intercostal (2.° EIC) permite detetar algum padrão anormal dada a maior proximidade com a CSVD.
MétodosEstudámos 18 doentes consecutivos submetidos a ablação de ESV da CSVD, idade média 44±16 anos, 12 mulheres, com coração aparentemente normal. O ECG foi obtido na posição standard e repetido com as derivações V1 e V2 a nível do 2 EIC. Foi efetuado mapa eletroanatómico de voltagem bipolar (MEV). Foram estabelecidos como área de baixa voltagem (ABV) os eletrogramas com amplitude <1.5 mV.
ResultadosO ECG no 2.° EIC não mostrou alterações em 11 doentes, mas em sete (39%) observou-se um padrão de supradesnivelamento do segmento ST (supra ST) em V1. O MEV revelou a presença de ABV em seis doentes (33%), a qual incluía a zona de aplicação em cinco doentes. O supra ST em V1 associou-se com a presença de ABV (p<0,0001) e com ABV no local de ablação (p=0,002).
ConclusõesNeste grupo de doentes com ESV da CSVD e coração aparentemente normal, a realização do ECG no 2.° EIC permitiu identificar a presença de supra ST em V1 em mais de um terço dos doentes que se associou com a presença de áreas de baixa voltagem na CSVD.
arrhythmogenic right ventricular cardiomyopathy
cardiac magnetic resonance imaging
earliest activation site
electrocardiogram
electroanatomical voltage mapping
intercostal space
low voltage area
magnetic navigation system
premature ventricular contraction
polymorphic ventricular tachycardia
right ventricular outflow tract
ventricular fibrillation
Premature ventricular contractions (PVCs) are a common finding in the normal population. The prognosis depends on the presence of structural heart disease; idiopathic PVCs are considered benign. Recently, however, evidence has emerged that a small percentage of patients may present with polymorphic ventricular tachycardia (PVT) or ventricular fibrillation (VF).1,2
Many potential markers have been studied, but none has so far been found that could predict this malignant outcome. It has been questioned whether all right ventricular outflow tract (RVOT) PVCs are in fact benign or whether some may represent an early stage of arrhythmogenic right ventricular cardiomyopathy (ARVC).
Initial studies with cardiac magnetic resonance imaging (cMRI) showed the presence of structural abnormalities, including localized wall bulging, focal wall thinning or fatty replacement in a high percentage of patients with PVCs and apparently normal hearts.3 However, most recent cMRI studies using modern techniques like electrocardiogram (ECG) gating and contrast-enhanced cMRI with delayed contrast enhancement used for imaging of ventricular scars have shown no pathological findings in patients with idiopathic RVOT PVCs.4
It has been demonstrated that the presence of myocardial fibrosis is associated with the occurrence of arrhythmic events. Myocardial fibrosis can be assessed noninvasively with cMRI or invasively with bipolar electroanatomical voltage mapping (EVM). However, the presence of fibrosis may reflect a more advanced form of the disease, and it would be useful to find other as yet unknown signs that are detectable in earlier stages of the disease.
We decided to perform an ECG in a higher position to assess whether closer proximity to the RVOT might expose features absent in the standard ECG that could help to identify patients who would present with abnormalities during the ablation procedure.
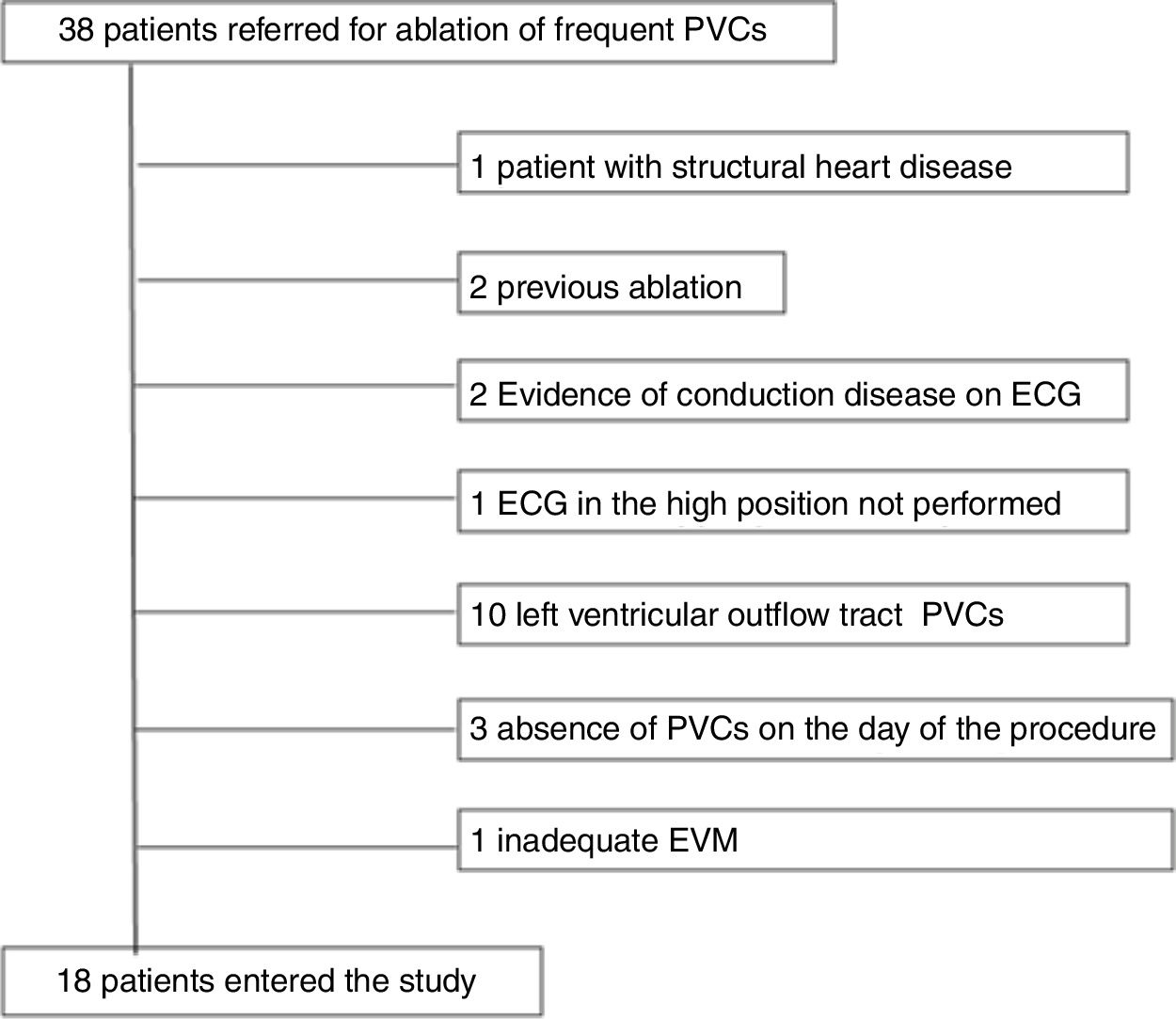
MethodsPatient selectionThis prospective study included consecutive patients undergoing mapping and ablation of frequent RVOT PVCs (defined as more than 10000/24 hours during Holter recording), refractory to medical therapy, between June 2014 and December 2016.
All patients underwent transthoracic echocardiography, including two-dimensional (2D), M-mode, and Doppler studies, and 12-lead ECG. When symptoms increased with exercise or when there was a suspicion the patient might have coronary heart disease, a treadmill exercise test was performed. cMRI was performed to rule out structural heart disease if any of the other exams were abnormal. Patients were excluded if structural heart disease was present, if they had undergone previous ablation or if the standard 12-lead ECG displayed evidence of conduction or electrical disease or abnormal QRS morphology. Patients with a PVC focus outside the RVOT were also excluded.
Study designDemographic data, cardiovascular risk factors, symptoms, transthoracic echocardiograms, 24-hour Holter monitoring and medications were recorded, as well as treadmill exercise test and cMRI results when performed. All patients underwent a standard 12-lead ECG and a 12-lead ECG in the second ICS.
Twelve-lead electrocardiogramAll ECGs were recorded in the electrophysiology laboratory on AXIOM Sensis™ (Siemens Systems) or EP-WorkMate 1.2.0™ (St. Jude Medical) recorders. Patients underwent a standard 12-lead ECG that included recordings of both premature ventricular and sinus rhythm beats using standard paper speed and calibration. The V1 and V2 leads were placed in the fourth ICS in the right and left sternal locations, respectively. The upper limb leads were placed on the respective shoulders, and the lower limb leads were placed on the respective side of the abdomen. The ECG was then repeated with the V1 and V2 leads placed in the second ICS, maintaining the other lead positions. The 12-lead ECG morphology of PVCs in both ECG positions and the morphology of the QRS and ST intervals in sinus rhythm were analyzed in the standard and higher positions. Right precordial T-wave inversion was considered present when beyond V1. The precordial transition in sinus rhythm and during PVCs was defined as the precordial lead in which the QRS changed from predominantly negative to predominantly positive and the R/S ratio became >1.
Mapping and ablationMapping technique and measurementsPatients were studied in a fasting non-sedated state. All antiarrhythmic drugs were discontinued at least five half-lives before the electrophysiological study.
Diagnostic catheters were positioned via the femoral vein with fluoroscopic guidance in the His position and in the great cardiac vein via the coronary sinus.
Isoprenaline was administered intravenously, as needed, and titrated to a dose that induced PVCs.
All patients underwent electroanatomical mapping by CARTO 3™ (Biosense Webster) or EnSite Velocity™ (St. Jude Medical) systems during sinus rhythm.
With CARTO 3 mapping, all procedures were performed using the Niobe™ magnetic navigation system (MNS) (Stereotaxis) working with the single-plane AXIOM Artis™ fluoroscopy system (Siemens) as previously described.5 An irrigated-tip Navistar RMT Thermocool™ catheter (Biosense Webster) was used with a 4-mm distal tip electrode and a 2-mm ring electrode with an interelectrode spacing of 1 mm.
With the EnSite Velocity, all procedures were performed manually with the BV Pulsera™ (Philips) single-plane fluoroscopy system and using a FlexAbility™ irrigated-tip catheter (St. Jude Medical) with a 4-mm distal tip electrode and 1-4-1 interelectrode spacing.
The ablation catheter was introduced via the femoral vein and advanced manually to the right atrium and then advanced to the His bundle and RVOT, automatically in the MNS patients and manually in the EnSite patients, under fluoroscopic guidance. The ablation catheter was then placed at multiple sites on the endocardial surface of the RVOT to record bipolar intracardiac electrograms (filtered at 30 to 400 Hz). This information was used to generate three-dimensional (3D) electroanatomical maps of the RVOT with the electrophysiological information color-coded and superimposed on the geometry.
Activation mappingThe activation map was created by mapping several points within the RVOT during each PVC, using a surface ECG lead as a reference. Activation times were assigned on the basis of the onset of bipolar electrograms and displayed as color gradients on a 3D activation map. Bipolar activation times were manually reviewed and the earliest isochrone was defined as the earliest activation site (EAS). After generation of isochrone reconstructions of the RVOT, bipolar pace mapping was performed at multiple endocardial sites near the EAS. Pacing was performed at cycle lengths as close as possible to that of the coupling interval of the PVC. The ablation site was selected based on the earliest endocardial activation time and confirmed by pace mapping that provided at least an 11 out of 12 match between paced and spontaneous PVCs.
Electroanatomical voltage mappingBipolar voltage reference for normal and abnormal myocardium was based on values validated by intraoperative and catheter mapping and used in previous voltage mapping studies.6
The color display for depicting normal and abnormal voltage myocardium ranged from red, representing electroanatomical scar tissue (amplitude <0.5 mV), to purple, representing electroanatomically normal tissue (amplitude >1.5 mV). Areas with signal amplitudes <1.5 mV represented low voltage areas (LVAs).
Complete endocardial maps were obtained to enable reconstruction of the 3D geometry of the RVOT.
Magnetic navigationThe MNS uses two computer-controlled permanent magnets positioned on either side of the fluoroscopy table. These magnets create a magnetic field of 0.1 T. The position of the magnets is remotely controlled by a console, the Navigant workstation, which changes the orientation of the magnetic field according to the vectors chosen by the operator. The ablation catheter has three magnets in its distal portion that render it parallel to the magnetic field. Changes in the orientation of the magnetic field deflect the catheter, which is remotely advanced or retracted with the aid of a computerized motor drive, Cardiodrive™ (Stereotaxis). Magnetic field vectors can be stored in order to automatically navigate the ablation catheter to previous sites.
AblationEnergy was delivered from an EP Shuttle™ radiofrequency generator (Stockert) between the distal electrode of the ablation catheter and a cutaneous patch for up to 120 s to a maximum temperature of 43°C and a power output limit of 35 W. When the application was ineffective, additional applications were delivered to sites adjacent to the EAS with a good pace-map.
During ablation, light sedation with midazolam (bolus) or remifentanil (continuous perfusion) was administered when needed.
Success was defined as elimination of PVCs under isoprenaline infusion for at least 30 min after ablation.
All patients were monitored in hospital for 24 hours after the procedure.
Follow-upFollow-up was performed at outpatient visits. Clinical assessment was carried out one to three months after ablation and regularly every six months thereafter. At least one 24-hour Holter recording was obtained after the procedure.
EthicsAll patients gave their written informed consent and the study was approved by the institutional review board. The study is in compliance with the Helsinki Declaration.
Statistical analysisIBM SPSS version 23 software (IBM SPSS Inc., Chicago, IL) was used for the statistical analysis. Data are expressed as means ± standard deviation for continuous variables and as frequencies and percentages for categorical variables. Baseline characteristics and outcomes were compared using the chi-square test for categorical variables and the t test for continuous variables. A value of p<0.05 was considered statistically significant.
ResultsStudy populationEighteen patients out of 38 were included in the study, mean age 45±16 years, 12 female (Figure 1). The patient characteristics are displayed in Table 1. Only one patient was asymptomatic; 17 patients complained of palpitations and one had a history of syncope, but none had documented episodes of sustained ventricular arrhythmias. Two patients had a family history of sudden death but none had a family history of ARVC. Physical examination, chest X-rays, and transthoracic echocardiography, including 2D, M-mode, and Doppler studies, were normal and demonstrated normal right ventricular size and function. Ten patients underwent treadmill exercise testing that showed a reduction of PVC frequency in nine patients and an increase in one, without evidence of ischemia. The 24-hour Holter recording showed a high PVC burden, with a mean of 24823±9546 PVC/24 hours. Seven patients underwent cMRI, three of them because of precordial T-wave inversion on the 12-lead ECG, to rule out ARVC, two because of a family history of sudden death, one because of PVC increase during the exercise test and one because of syncope. The cMRI was normal in all. A flecainide test was administered to the patient with ST elevation and syncope to rule out Brugada syndrome, which was negative.
Baseline characteristics of the study groups.
| Overall sample (n=18) | Normal second ICS ECG (n=11) | ST elevation second ICS (n=7) | p | |
|---|---|---|---|---|
| Demographic data | ||||
| Male gender, n (%) | 6 (33) | 4 (36) | 2 (28) | NS |
| Age, years, mean ± SD | 44.7±15.7 | 48±12.7 | 42.6±17.6 | NS |
| Risk factors and history | ||||
| Family history of sudden death, n (%) | 2 (11) | 2 (18) | 0 | NS |
| Absence of risk factors, n (%) | 14 (77) | 9 (82) | 5 (71) | NS |
| Strenuous exercise, n (%) | 1 (5.6) | 1 (14) | 0 | NS |
| Symptoms | ||||
| Asymptomatic, n (%) | 1 (5.6) | 0 | 1 (14) | NS |
| Syncope, n (%) | 1 (5.6) | 0 (0) | 1 (14) | NS |
| Palpitations, n (%) | 17 (94.4) | 11 (100) | 6 (86) | NS |
| Previous sustained VT, n (%) | 0 | 0 | 0 | NS |
| Duration of symptoms, months | 40±30 | 45±30 | 32.2±31.1 | NS |
| Medications | ||||
| Beta-blockers, n (%) | 14 (77.8) | 8 (72) | 6 (86) | NS |
| Antiarrhythmics, n (%) | 2 (11.1) | 2 (18) | 0 | NS |
| Standard 12-lead ECG | ||||
| Right precordial T-wave inversion, n (%) | 3 (16.7) | 0 | 3 (43) | 0.043 |
| Sinus rhythm precordial transition at V3, n (%) | 2 (11) | 0 | 2 (28) | NS |
| Sinus rhythm precordial transition beyond V3, n (%) | 16 (88) | 11 (100) | 5 (71) | NS |
| PVC precordial transition at V3, n (%) | 4 (22) | 2 (18) | 2 (28) | NS |
| PVC precordial transition beyond V3, n (%) | 14 (77) | 9 (82) | 5 (71) | NS |
| QRS duration (ms) | 86.8±8.3 | 85.9±6.9 | 88.4±10 | NS |
| Treadmill exercise test (n=10) | ||||
| Exercise-induced increase in PVC frequency, n (%) | 1 (10) | 1 (16) | 0 (0) | NS |
| Exercise-induced reduction in PVC frequency, n (%) | 9 (90) | 5 (83) | 4 (100) | NS |
| 24-hour Holter recording | ||||
| No. of PVCs/24 hours | 24 823±9546 | 24 274±10649 | 25 109±8297 | NS |
| NSVT, n (%) | 5 (27.8) | 3 (27) | 2 (28) | NS |
| cMRI (n=7) | ||||
| Normal, n (%) | 7 (100%) | 3 (27) | 4 (57) | NS |
cMRI: cardiac magnetic resonance imaging; ECG: electrocardiogram; ICS: intercostal space; NSVT: non-sustained ventricular tachycardia; PVC: premature ventricular contraction; SD: standard deviation; VT: ventricular tachycardia.
The precordial transition in sinus rhythm was beyond V3 in all patients, as was the precordial transition of the PVCs. In two patients the transition of the PVC was later than the transition in sinus rhythm, but in 16 patients it was earlier or in the same precordial lead.
Three patients had T-wave inversion in V1 to V3, but the ECG was otherwise normal in all.
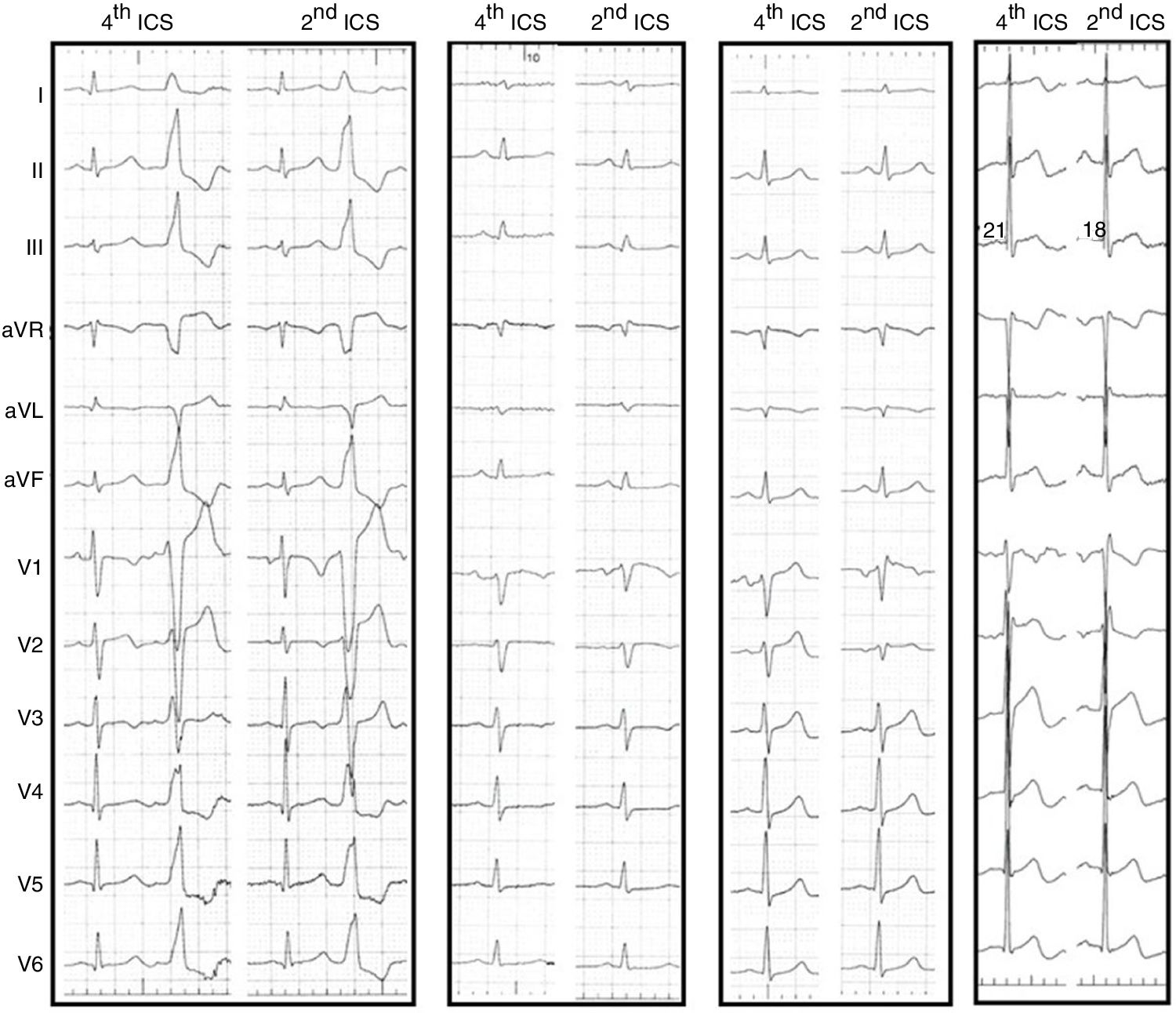
Second intercostal space electrocardiogramThe upward shift of the precordial leads V1 and V2 to the second ICS resulted in the appearance of ST-segment elevation in seven patients (39%). The ST elevation was less than 2 mm, convex and followed by a negative T wave, but did not reach criteria for type 1 Brugada pattern (Figure 2). Five patients (28%) had an rSr’ pattern in V1, not present on the standard ECG, and in two patients both features were present (Figure 2).
12-lead electrocardiogram (ECG) of four different patients comparing the tracings with V1 and V2 in the standard and high positions. From left to right, in the first patient V1 does not change, the PVC loses R in V1 and V2 in the second ICS. In the second patient the upper ECG displays ST elevation in V1, in the third patient ST elevation with rSr’ pattern and in the fourth patient an rSr’ pattern without ST elevation. ICS: intercostal space; PVC: premature ventricular contraction.
The pattern of the PVC changed little, becoming more negative with the loss of the initial r in leads V1 and V2, changing from rS to QS.
The clinical and non-invasive data for both groups with and without ST-segment elevation are displayed in Table 1. We found no differences except for a higher frequency of precordial T-wave inversion in the group with ST elevation (0% vs. 43%, p=0.043).
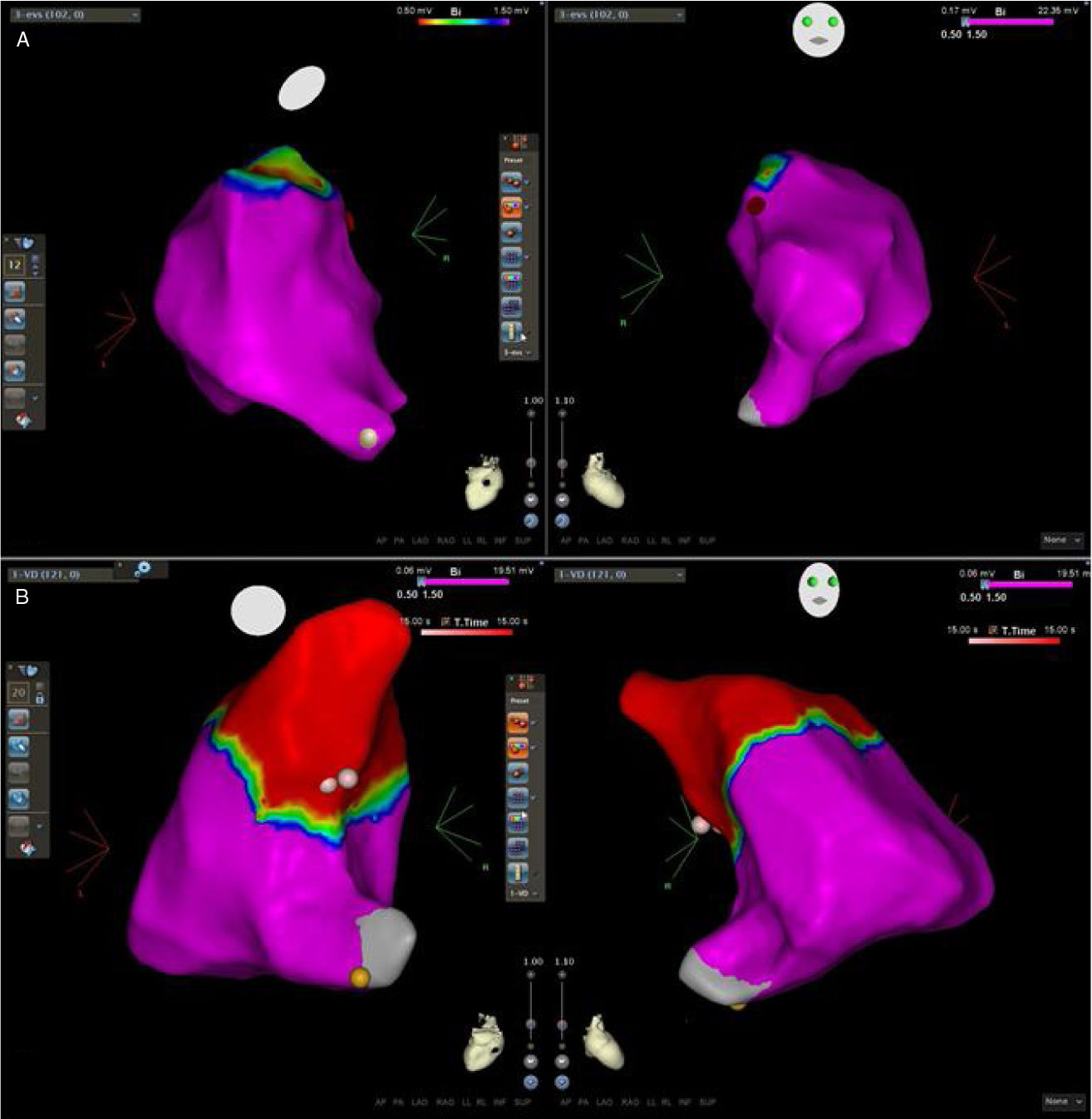
Mapping and ablationElectroanatomical mapping was successfully acquired in all patients. The mean number of points used to obtain the RVOT map was 78±29. The activation map identified the EAS in the RVOT free wall in seven patients and in the RVOT septum in 11. In all patients there was an LVA at the level of the pulmonary valve. However, in six patients (33%) the LVA extend more proximally and in five patients the EAS was within the LVA (Figure 3). The acute success rate was 89% and there were no complications. The two patients with unsuccessful ablation are being followed on an outpatient basis and despite continuing to experience a high number of PVCs/24 hours, refused a second procedure.
Three-dimensional electroanatomical voltage maps of the RVOT. (A): A patient with normal voltage map; (B): a patient with an LVA extending into the RVOT. Pink and red dots indicate the successful ablation site and yellow dots indicate the His bundle. Note that the ablation site lies within the LVA in the second patient. LVA: low voltage area; RVOT: right ventricular outflow tract.
We found an association between ST-segment elevation in the higher ECG and the presence of LVA (86% vs. 0% vs. p<0.0001) and the presence of EAS in the LVA (71% vs. 0%, p=0.002) (Table 2). The presence of an rSr’ pattern did not correlate with the presence of LVA (2/5 [40%] vs. 4/13 [31%] (NS). The presence of LVAs or ST elevation did not correlate with acute success.
Mapping, ablation and follow-up data.
| Overall sample (n=18) | Normal second ICS ECG (n=11) | ST-elevation second ICS (n=7) | p | |
|---|---|---|---|---|
| Mapping data | ||||
| Mapping system (CARTO/NavX), n | 11/7 | 8/3 | 3/4 | NS |
| Number of points | 78±29 | 75±27 | 83.7±33.6 | NS |
| EAS RVOT free wall, n (%) | 7 (39) | 4 (36) | 3 (43) | NS |
| EAS RVOT septum, n (%) | 11 (61) | 7 (64) | 4 (57) | NS |
| Presence of LVAs, n (%) | 6 (33) | 0 | 6 (86) | <0.0001 |
| Ablation data | ||||
| EAS in the LVA, n (%) | 5 (28) | 0 | 5 (71) | 0.002 |
| Acute success, n (%) | 16 (89) | 9 (82) | 7 (100) | NS |
| Complications, n (%) | 0 | 0 | 0 | NS |
| Follow-up data | ||||
| Duration of follow-up (months) | 19.5±9.6 | 22±11 | 15.7±6.5 | NS |
| Recurrence of PVCs, n (%) | 0 | 0 | 0 | NS |
| No. of PVCs on 24-hour Holter | 21±31 | 16±6 | 37±45 | NS |
| Beta-blockers, n (%) | 1 (6) | 0 | 1 (14) | NS |
| Antiarrhythmics, n (%) | 0 | 0 | 0 | NS |
EAS: earliest activation site; ICS: intercostal space; LVA: low voltage area; PVC: premature ventricular contraction; RVOT: right ventricular outflow tract.
During a mean follow-up of 19.5±9.6 months, in the 16 patients in whom ablation was successful there were no recurrences. All patients remained free of symptoms. Antiarrhythmic drugs were suspended after ablation and only one patient continued beta-blocker therapy, for hypertension. All patients underwent 24-hour Holter recording and there was no recurrence of the ablated PVCs; in some cases there were still occasional PVCs, but with a different morphology. The number of PVCs/24 hours was dramatically reduced, from 24823±9546 to 21±31.
DiscussionIn the absence of structural heart disease, PVCs have generally been considered to have a benign prognosis.7 However, recently there have been reports suggesting that a small percentage of patients may present with PVT or VF. Viskin et al.1 reported the clinical history of three patients with apparently benign idiopathic PVCs of the RVOT who had episodes of PVT or VF during follow-up. Noda et al.2 studied 16 out of 101 patients without structural heart disease who underwent ablation of RVOT PVCs and presented during follow-up with episodes of PVT or VF induced by PVCs.
It is important to assess whether the entity known as idiopathic RVOT PVCs may affect patients with some form of concealed structural heart disease at a very early stage, and if so, to identify the features that suggest that the patient may have a worse prognosis.
The specific marker of ARVC is the transmural loss of right ventricular myocardium and the presence of fibrofatty deposition is no longer considered diagnostic.8 This loss of cardiac cells results in the appearance of areas of low voltage and electrical scar on EVM.9,10 However, the results of EVM in patients with idiopathic RVOT PVCs are contradictory. In our study we demonstrated the presence of LVAs in the RVOT in 33% of patients. Boulos et al.9 obtained different results. They compared the EVM of patients with idiopathic RVOT tachycardia to that of patients with ARVC and normal controls and concluded that patients with idiopathic VT and normal controls had similar EVM without LVAs, unlike the ARVC patients, who presented with LVAs. Other authors likewise demonstrated the presence of these LVAs in patients with idiopathic RVOT arrhythmias. Furushima et al.11 performed EVM in 28 patients with apparently normal hearts and found LVAs immediately below the pulmonary valve in all patients, the width of the LVA being variable. Two patients had PVT/VF, and these were the ones with wider LVAs, although these patients did not undergo further investigation to rule out ARVC. The authors conclude that in the presence of a wider LVA, its potential arrhythmogenic impact must be borne in mind.
Corrado et al.6 studied 27 patients out of 89 with RVOT arrhythmias and apparently normal hearts by EVM and endomyocardial biopsy. They found LVAs in seven patients (26%), an incidence very close to ours. Although without criteria for the diagnosis of ARVC according to the authors, these patients had symptoms or signs suggestive of potential structural disease, and yet only seven underwent cMRI, which was diagnostic of ARVC in four.
In our study, patients in whom structural heart disease was suspected due to T-wave inversion, syncope, family history of sudden death or worsening of arrhythmia during exercise underwent cMRI, which excluded ARVC.
EVM is an invasive procedure requiring cardiac catheterization, so it cannot be proposed as a routine method to assess patients with PVCs unless there is an indication for catheter ablation.
Recently, it has been demonstrated that upward displacement of leads V1 and V2 to the second ICS can improve the sensitivity of Brugada syndrome diagnosis due to the greater proximity to the RVOT.12
We decided to perform the ECG in the higher position since we were mapping the RVOT. In this position we identified a pattern of ST-segment elevation with or without an rSr’ pattern that is absent in the standard ECG. The presence of an rSr’ pattern has been described in the upper position in normal individuals.13 Previous studies have also shown the presence of ST-segment elevation in V1-V2 at the second ICS identified as a Brugada type 2 or 3 pattern in athletes,14 normal Asiatic populations15 and normal European populations.16 In these studies the reported incidence of ST elevation varies from 1.3%15 to 4.3%16 excluding the type 3 Brugada pattern. Chung et al.14 found a higher incidence (12%), but they included the Brugada type 3 pattern, which is different from the morphology of our ST elevation, which was coved, although not reaching the criteria for Brugada type 1. All these studies showed a significantly lower incidence of ST elevation in the higher ECG than the 39% in our study, and it is important to underline that in these studies the subjects were not studied with cMRI to rule out structural heart disease.
The major finding of our study was the association between the presence of LVAs and ST-segment elevation in V1 in the ECG performed at the second ICS in patients with apparently normal hearts. To the best of our knowledge this is the first report of this association. Patients with ST elevation showed similar baseline characteristics to those without, with the exception of a higher incidence of T-wave inversion, but these patients underwent cMRI that ruled out the presence of ARVC.
The meaning of this ST elevation, and of the presence of LVA, remains unknown, however there seems to be an association between the two.
We do not know the long-term prognostic implications of these findings, but we found no impact on the acute success rate or short-term follow-up.
Krittayaphong et al.,17 on the contrary, demonstrated that the presence of abnormalities on cMRI in patients with RVOT arrhythmias referred for catheter ablation without diagnostic criteria for ARVD was associated with a lower success rate and higher recurrence.
Although previous trials have demonstrated a correlation between the presence of scar on cMRI and on EVM,18 they mostly involved the left ventricle. In the right ventricle, EVM demonstrated a higher sensitivity than cMRI for a diagnosis of LVAs.19
The definition of a condition as idiopathic is based on the absence of abnormalities in diagnostic tests and on lack of knowledge of its cause, but it does not imply absence of pathological abnormalities. In fact, Letsas et al.20 recently demonstrated the presence of LVAs in the RVOT endocardium of asymptomatic type 1 Brugada patients using high-density endocardial voltage mapping despite the absence of delayed contrast enhancement on cMRI.
Thus we may speculate that in RVOT arrhythmias the presence of LVAs may be a marker of a very early stage of disease, not detected by current imaging techniques, and that ST elevation in V1 at the second ICS may be a surrogate marker of these LVAs.
Study limitationsThe number of patients in our study was relatively small and there was no control group. cMRI was performed in only a small percentage of patients because it is not a routine examination for patients with PVC and apparently normal hearts, and when performed it was not repeated during follow-up.
EVM was performed with two different systems with different methodologies. However, the electrogram analysis is similar and the threshold for defining low voltage is the same in both, and this has been validated previously. The mean follow-up time of 19 months is very short to assess the long-term success of the procedure and to demonstrate the benign outcome in this population.
ConclusionIn this group of patients with apparently normal hearts and with frequent PVCs of the RVOT, EVM during the ablation procedure displayed the presence of LVAs. Upward displacement of the ECG showed the presence of ST elevation in V1 in more than one third of patients, and this ST elevation was associated with the presence of LVAs in the RVOT.
Neither ST-segment elevation nor the presence of LVAs predicted the success or recurrence rate.
Conflicts of interestThe authors have no conflicts of interest to declare.




 ECG: electrocardiogram;
ECG: electrocardiogram; 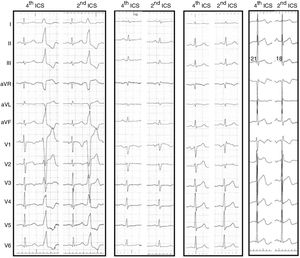 ECG) of four different patients comparing the tracings with V1 and V2 in the standard and high positions. From left to right, in the first patient V1 does not change, the
ECG) of four different patients comparing the tracings with V1 and V2 in the standard and high positions. From left to right, in the first patient V1 does not change, the 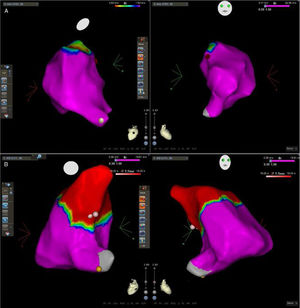 RVOT. (A): A patient with normal voltage map; (B): a patient with an
RVOT. (A): A patient with normal voltage map; (B): a patient with an 
