Current clinical guidelines for ST-segment elevation myocardial infarction (STEMI) suggest prehospital activation of the cardiac catheterization team. In previous protocols in our center activation occurred once patients arrived at the hospital. In January 2011, we initiated a new primary angioplasty activation protocol from prehospital locations. Our objective was to quantify the influence of this change on reperfusion times.
MethodsA total of 173 consecutive STEMI patients (n=73/100 before/after initiation of the new protocol), diagnosed in a prehospital setting within 12 hours of symptom onset, were analyzed. The time between the patient's arrival at the hospital and beginning of the angioplasty procedure was termed the cath lab activation delay.
ResultsThe new protocol resulted in a 37-min reduction in system delay (166 [132–235] min before vs. 129 [105–166] min after, p<0.001), mostly driven by a 64% reduction in cath lab activation delay (55 [0–79] min before vs. 20 [0–54] min after, p=0.001). This reduction was mainly observed outside working hours. The percentage of patients treated with a system delay ≤120 min increased from 14.5% before the new protocol to 41.8% afterwards (p=0.001).
ConclusionsPrehospital activation of the cardiac catheterization team resulted in earlier reperfusion of STEMI patients.
As atuais diretrizes clínicas aquando da ocorrência de um enfarte agudo miocárdio com elevação do segmento ST (STEMI) sugerem a ativação da equipa de angioplastia primária ao nível pré-hospitalar. Protocolos anteriores contemplam a ativação da referida equipa assim que os pacientes chegam ao hospital. Em janeiro de 2011, o nosso centro iniciou um novo protocolo de ativação da equipa de angioplastia primária em localização pré-hospitalar de modo a quantificar a influência de tal alteração nos tempos de reperfusão.
MétodosForam analisados 173 pacientes consecutivos com STEMI, cujo diagnóstico se efetuou em local pré-hospitalar em 12 horas desde o início dos sintomas (n = 73/100 antes/ após início do novo protocolo). O tempo que decorreu entre a chegada do paciente ao hospital e o inicio do procedimento de angioplastia foi designado Cath Lab Activation Delay.
ResultadosO novo protocolo refletiu uma redução de 37 minutos no System Delay (166 [132 – 235] antes versus 129 [105 – 166] minutos depois, p<0.001), que se deveu primordialmente à redução de 64% no Cath Lab Activation Delay (55 [0 – 79] minutos antes versus 20 [0 – 54] minutos depois, p = 0,001). Tal redução observou-se principalmente em horário pós-laboral. A percentagem de pacientes tratados com um System Delay ≤ 120 minutos aumentou de 14,5%, antes do início do novo protocolo, para 41,8% após (p = 0,001).
ConclusõesA ativação da equipa de angioplastia primária ao nível pré-hospitalar permitiu uma maior celeridade no início da terapia de reperfusão em pacientes com STEMI.
cath lab activation delay
door-to-balloon
emergency medical services
first medical contact
left ventricular ejection fraction
percutaneous coronary intervention
system delay
ST-segment elevation myocardial infarction
Treatment of ST-segment elevation myocardial infarction (STEMI) is based on early reperfusion therapy.1,2 A meta-analysis by Keeley et al. showed the superiority of primary percutaneous coronary intervention (PCI) over fibrinolysis in terms of mortality, reinfarction and bleeding risk.3 Current clinical guidelines stipulate that primary PCI is the preferred reperfusion therapy as long as it can be performed within 120 min of first medical contact (FMC) with the patient.1,2,4 This time interval (FMC-reperfusion) is called system delay (SD) and is directly related to prognosis.5
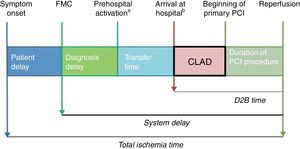
SD can be separated into various time intervals (Figure 1): diagnosis delay or time from FMC to an electrocardiogram showing diagnostic criteria of STEMI; the time taken to transfer the patient to a PCI-capable center (when FMC occurs outside a PCI-capable center); time from arrival at the PCI-capable center to beginning of PCI in the catheterization laboratory; and the duration of primary PCI, until coronary reperfusion is achieved. The sum of these two last time intervals is termed door-to-balloon (D2B) time.
Components of ischemia time. CLAD: cath lab activation delay; D2B time: door-to-balloon time; FMC: first medial contact; PCI: percutaneous coronary intervention.
a Since initiation of the new primary PCI protocol, the cardiac catheterization team is activated from the FMC site by a single phone call.
b Before initiation of the new primary PCI protocol, the cardiac catheterization team was activated on the patient's arrival at our hospital.
The previous primary PCI protocol in our center activated the cardiac catheterization team after the patient had arrived at the hospital and the on-duty cardiology team had confirmed the diagnosis of STEMI. During normal working hours (8 am-10 pm, Monday to Friday), there is a cardiac catheterization team ready at the hospital, while after hours the cardiac catheterization team is at home. Therefore, patients presenting after hours had to wait until the cardiac catheterization team arrived at the hospital for primary PCI to be initiated.
In January 2011, a new primary PCI activation protocol was implemented in our hospital, establishing that the cardiac catheterization team would be activated by a single phone call from the FMC site: by the emergency medical services (EMS) if FMC occurred outside a medical center, or by the medical team who diagnosed STEMI if FMC occurred inside a medical center. Current clinical guidelines2 suggest this protocol, albeit without a clear recommendation.
This new protocol was intended to minimize SD by reducing the time from patients’ arrival at our center to the beginning of primary PCI, as early activation of the cardiac catheterization team could allow them to arrive at the hospital and the catheterization laboratory to be prepared while the patient is being transferred. We termed this time interval (patient's arrival at the hospital to beginning of primary PCI) the cath lab activation delay (CLAD).
Our primary objective was to determine whether the new primary PCI activation protocol has achieved its goal of reducing SD, by reducing CLAD, in STEMI patients treated with primary PCI in our hospital. In addition, the percentage of patients meeting the treatment delay targets established by the clinical guidelines1,2 was measured. Secondary objectives were to analyze the differences in SD between “working-hours” patients and “after-hours” patients, and between patients with different FMC locations.
MethodsStudy populationThe new primary PCI activation protocol took effect on January 20, 2011. All patients with a diagnosis of STEMI treated by primary PCI in our hospital in the previous six months and the following six months (total study duration: one year) were included (n=241). Before January 20, 2011, all patients were treated following the previous PCI protocol, and after that date, all patients were treated according to the new protocol. Patients with FMC in our hospital (n=36) were excluded from the analysis, as the new protocol did not affect them. Also, patients with more than 12 hours from symptom onset to FMC were excluded (n=32). Hence, a total of 173 patients were included in the analysis. The study complied with the ethical guidelines of the 1975 Declaration of Helsinki and received prior approval by the human research committee of our institution (Hospital Clínico San Carlos, Madrid, Spain).
Measures and data collectionPatients’ demographic and clinical data were prospectively recorded during their stay in our hospital, and retrospectively analyzed after the study period. The time variables obtained were: time of symptom onset; time of FMC; time of STEMI diagnosis (according to the time at which the first electrocardiogram meeting STEMI criteria was recorded); time of patient's arrival at our hospital; time of beginning of primary PCI; time of culprit artery reperfusion.
Every patient was transferred directly to the catheterization laboratory on arrival at our hospital (both before and after initiation of the new protocol), if the catheterization laboratory was ready and the cardiac catheterization team was present. If this was achieved, CLAD was considered to be zero. Otherwise, patients were admitted to the coronary care unit until the catheterization laboratory and cardiac catheterization team were ready.
DefinitionsA diagnosis of STEMI was based on the presence of symptoms of myocardial ischemia, associated with new persistent ST-segment elevation or left bundle branch block, according to current clinical guidelines1,2,6 and confirmed by detection of a rise in cardiac biomarkers (creatine kinase and/or troponin) above the 99th percentile of the upper reference limit.
Chronic renal failure was defined as a glomerular filtration rate of less than 60 ml/min/1.73 m2 in at least two blood tests obtained at an interval of more than three months, which corresponds to category G3 or higher chronic kidney disease according to the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) classification.7
Statistical analysisCategorical variables were expressed as number (percentage) and compared by the chi-square test or Fisher's exact test, as appropriate. Continuous variables were expressed as mean ± standard deviation (SD) for variables with normal distribution, or as median [interquartile range] for non-normal variables. The Kolmogorov-Smirnov test was used to assess normality in continuous variables. Comparisons between normal continuous variables were made using the Student's t test (comparisons between two groups) or ANOVA (comparisons between more than two groups); for non-normal variables, the Mann-Whitney U test (comparisons between two groups) and the Kruskal-Wallis test (comparisons between more than two groups) were used. A two-tailed p of <0.05 was considered statistically significant.
The statistical analysis was performed using SPSS PASW Statistics version 15.0 (SPSS Inc., Chicago, Ill, USA).
ResultsA total of 173 consecutive STEMI patients, with FMC outside our center and less than 12 hours from symptom onset to FMC, were treated by primary PCI in our hospital during the study period, of whom 73 (42.1%) were treated before the new protocol implementation, and 100 (57.8%) were treated afterwards. The demographic and clinical characteristics of these patients are shown in Table 1. There were no significant differences between the two groups (patients treated before and after the new protocol) in factors that could delay primary PCI, such as age or Killip class IV at admission.
Demographic and clinical characteristics of the study population according to presentation before and after the new primary PCI protocol.
| Total | Before new primary PCI protocol | After new primary PCI protocol | p | |
| Number of patients (%) | 173 | 73 (42.1) | 100 (57.8) | |
| Demographic characteristics | ||||
| Age (mean, SD) | 61.8, SD=14.2 | 63.7, SD=14.4 | 60.5, SD=14.0 | 0.146 |
| Male (%) | 137 (79.2) | 54 (74.0) | 83 (83.0) | 0.149 |
| Cardiovascular risk factors | ||||
| Hypertension (%) | 97 (56.1) | 51 (69.9) | 46 (46.0) | 0.002 |
| Diabetes (%) | 31 (17.9) | 15 (20.5) | 16 (16.0) | 0.359 |
| Hypercholesterolemia (%) | 73 (42.2) | 34 (46.6) | 39 (39.0) | 0.319 |
| Obesity (%) | 25 (14.5) | 9 (12.3) | 16 (16.0) | 0.498 |
| Smoking (%) | 112 (64.7) | 43 (58.9) | 69 (69.0) | 0.327 |
| Current | 77 (68.8) | 31 (72.1) | 46 (66.7) | |
| Former | 35 (31.2) | 12 (27.9) | 23 (33.3) | |
| Chronic renal failure (%) | 10 (5.8) | 8 (10.9) | 2 (2.0) | 0.036 |
| Family history (%) | 8 (4.6) | 4 (5.5) | 4 (4.0) | 0.723 |
| Previous MI (%) | 16 (9.2) | 10 (13.7) | 6 (6.0) | 0.084 |
| Clinical presentation | ||||
| Killip class IV on presentation (%) | 7 (4.0) | 4 (5.5) | 3 (3.0) | 0.457 |
| STEMI location | ||||
| Anterior/lateral (%) | 90 (52.0) | 39 (53.4) | 51 (51.0) | 0.304 |
| Inferior (%) | 78 (45.1) | 30 (41.1) | 48 (48.0) | |
| Undetermined (%) | 5 (2.9) | 4 (5.5) | 1 (1.0) | |
MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction.
Regarding cardiovascular risk factors, patients treated before initiation of the new protocol had a higher prevalence of hypertension (69.9% vs. 46.0%, p=0.002) and chronic renal failure (10.9% vs. 2.0%, p=0.036). The prevalence of other cardiovascular risk factors was similar between the two groups.
Primary objectiveA significant 37-min reduction in SD was observed after initiation of the new primary PCI protocol (from 166 [132–235] min to 129 [105–166] min, p<0.001) (Figure 2). CLAD was reduced by 35 min (from 55 [0–79] min to 20 [0–54] min, p=0.001), a 64% relative reduction. D2B time was also improved (absolute reduction of 34 min, p=0.005).
There were no significant differences between the groups in the time between FMC and patient arrival at our hospital (diagnosis delay plus transfer time), or in the duration of the primary PCI procedure (Table 2).
Analysis of ischemia time components and system delay before and after initiation of the new primary PCI protocol.
| Before primary PCI protocol | After primary PCI protocol | p | |
| Total ischemia time | |||
| Total ischemia time | 245 [189–360] | 227 [180–371] | 0.440 |
| Patient delay (symptom onset to FMC) | 63 [35–120] | 90 [59–199] | 0.058 |
| SD | |||
| Total SD | 166 [132–235] | 129 [105–166] | <0.001 |
| Sum of diagnosis delay plus transfer | 93 [65–163] | 74 [60–123] | 0.119 |
| CLAD | 55 [0–79] | 20 [0–54] | 0.001 |
| Duration of primary PCI procedure | 20 [14–29] | 20 [15–26] | 0.972 |
| D2B time | 77 [35–96] | 43 [20–73] | 0.005 |
| Clinical guidelines goals | |||
| SD ≤120 min (%) | 14.5% | 41.8% | 0.001 |
| CLAD=0 (%) | 28.1% | 44.0% | 0.035 |
CLAD: cath lab activation delay; D2B time: door-to-balloon time; FMC: first medial contact; PCI: percutaneous coronary intervention; SD: system delay. Data are shown as median [interquartile range].
Current clinical guidelines1,2 establish a ≤120 min target for SD. The percentage of patients meeting this objective improved from 14.5% before implementation of the new protocol to 41.8% afterwards (p=0.001).
Another recommendation of the guidelines is that every STEMI patient with FMC outside a PCI-capable hospital should be transferred directly to the catheterization laboratory on arrival, without previous admission to any other unit (emergency department, coronary care unit, etc.). If this objective is met, we define CLAD=0. The percentage of patients meeting this objective of CLAD=0 rose significantly from 28.1% before initiation of the new primary PCI protocol to 44.0% afterwards (p=0.035).
Comparison between working-hours and after-hours patientsThe new primary PCI protocol is especially valuable after hours, as it allows the cardiac catheterization team to arrive at the hospital while the patient is being transferred. The percentage of after-hours patients admitted before (56.2%) and after (60.0%) the new protocol implementation was similar (p=0.613).
After hours, significant reductions of 41 min in CLAD (p<0.001), 34 min in D2B time (p=0.005) and 24 min in SD (p=0.005) were noted after initiation of the new protocol (Figure 3). The percentage of patients treated with SD ≤120 min improved from 0% to 36.6% (p<0.001), and the percentage of patients transferred directly to the catheterization laboratory on arrival (CLAD=0) almost tripled (from 15.0% to 43.3%, p=0.016). Treatment delays did not change significantly in working-hours patients, although a non-significant trend to improvement was noted (Table 3).
Analysis of ischemia time components and system delay before and after initiation of the new primary PCI protocol: working-hours vs. after-hours patients.
| Working hours | Before primary PCI protocol initiation | After primary PCI protocol initiation | p |
| Total ischemia time | |||
| Total ischemia time | 223 [165–398] | 226 [161–361] | 0.911 |
| Patient delay (symptom onset to FMC) | 60 [32–90] | 90 [59–187] | 0.050 |
| SD | |||
| Total SD | 151 [114–309] | 125 [102–162] | 0.089 |
| Sum of diagnosis delay plus transfer | 87 [41–176] | 70 [60–104] | 0.469 |
| CLAD | 31 [0–80] | 19 [0–49] | 0.407 |
| Duration of primary PCI procedure | 24 [16–32] | 20 [16–26] | 0.258 |
| D2B time | 59 [21–108] | 41 [23–67] | 0.444 |
| Clinical guidelines goals | |||
| SD ≤120 min (%) | 33.3% | 47.3% | 0.165 |
| CLAD=0 (%) | 45.2% | 45.0% | 0.409 |
| After hours | Before primary PCI protocol initiation | After primary PCI protocol initiation | p |
| Total ischemia time | |||
| Total ischemia time | 290 [213–356] | 232 [189–411] | 0.237 |
| Patient delay (symptom onset to FMC) | 90 [35–128] | 90 [48–240] | 0.454 |
| SD | |||
| Total SD | 170 [144–235] | 146 [109–171] | 0.005 |
| Sum of diagnosis delay plus transfer | 95 [75–147] | 79 [60–136] | 0.222 |
| CLAD | 63 [28–78] | 22 [0–59] | <0.001 |
| Duration of primary PCI procedure | 19 [14–25] | 19 [15–26] | 0.420 |
| D2B time | 78 [41–96] | 44 [17–79] | 0.005 |
| Clinical guidelines goals | |||
| SD ≤120 min (%) | 0% | 36.6% | <0.001 |
| CLAD=0 (%) | 15% | 43.3% | 0.016 |
CLAD: cath lab activation delay; D2B time: door-to-balloon time; FMC: first medial contact; PCI: percutaneous coronary intervention; SD: system delay. Data are shown as median [interquartile range].
Before initiation of the new protocol, after-hours patients were less likely to be treated within 120 min of SD (p=0.001) or to be transferred directly to the catheterization laboratory on arrival (p=0.005) than working-hours patients. After initiation of the new primary PCI protocol, these differences disappeared.
Analysis of treatment delays according to location of first medical contactOur last objective was to analyze the differences between patients whose FMC had occurred in different locations (EMS, walk-in clinics and non-PCI-capable hospitals). The results are shown in Table 4.
Analysis of ischemia time components and system delay before and after initiation of the new primary PCI protocol, according to location of first medical contact.
| Before new primary PCI protocol | EMS | Walk-in clinics | Non-PCI-capable hospitals | p |
| Number of patients (%) | 43 (58.9) | 10 (13.7) | 20 (27.4) | |
| Total ischemia time | ||||
| Total ischemia time | 215 [178–295] | 280 [226–570] | 424 [354–537] | <0.001 |
| Patient delay (symptom onset to FMC) | 60 [34–90] | 91 [40–558] | 105 [6–169] | 0.212 |
| SD | ||||
| Total SD | 147 [125–170] | 199 [159–235] | 280 [213–419] | <0.001 |
| Sum of diagnosis delay plus transfer | 78 [46–107] | 93 [83–120] | 176 [135–302] | 0.001 |
| CLAD | 51 [15–78] | 23 [0–77] | 63 [0–81] | 0.530 |
| Duration of primary PCI procedure | 20 [14–27] | 22 [16–36] | 21 [15–30] | 0.544 |
| D2B time | 70 [35–94] | 50 [22–102] | 86 [66–110] | 0.323 |
| Clinical guidelines goals | ||||
| SD ≤120 min (%) | 17.1% | 25.0% | 0% | 0.230 |
| CLAD=0 (%) | 23.8% | 40.0% | 31.5% | 0.550 |
| After new primary PCI protocol | EMS | Walk-in clinics | Non-PCI-capable hospitals | p |
| Number of patients (%) | 71 (71.0) | 12 (12.0) | 17 (17.0) | |
| Total ischemia time | ||||
| Total ischemia time | 214 [176–297] | 212 [151–425] | 448 [349–588] | 0.001 |
| Patient delay (symptom onset to FMC) | 90 [48–160] | 68 [60–300] | 180 [100–300] | 0.426 |
| SD | ||||
| Total SD | 125 [104–160] | 131 [112–197] | 315 [175–511] | 0.003 |
| Sum of diagnosis delay plus transfer | 71 [60–105] | 60 [50–129] | 180 [134–349] | 0.007 |
| CLAD | 24 [0–57] | 30 [3–54] | 0 [0–45] | 0.483 |
| Duration of primary PCI procedure | 19 [15–27] | 22 [20–25] | 21 [17–26] | 0.332 |
| D2B time | 44 [18–78] | 58 [27–90] | 32 [22–58] | 0.395 |
| Clinical guidelines goals | ||||
| SD ≤120 min (%) | 46.0% | 36.4% | 0% | 0.123 |
| CLAD=0 (%) | 43.7% | 33.3% | 52.9% | 0.574 |
CLAD: cath lab activation delay; D2B time: door-to-balloon time; EMS: emergency medical services; FMC: first medial contact; PCI: percutaneous coronary intervention; SD: system delay. Data are shown as median [interquartile range].
The location of FMC for patients treated before and after initiation of the new primary PCI protocol did not differ significantly (p=0.205). In both groups (before and after new protocol implementation), longer SD (p<0.001) and total ischemia time (p<0.001) were observed in patients with FMC in non-PCI-capable hospitals (Figure 4). This was related to a longer transfer time (p<0.001). No differences were noted in treatment delays once patients arrived at our hospital (D2B time, CLAD).
Importantly, no patient transferred from a non-PCI-capable hospital achieved SD ≤120 min.
Infarct sizeInfarct size, estimated on the basis of peak creatine kinase and troponin I levels obtained in each patient, was compared before and after initiation of the new primary PCI protocol. Non-significant reductions of 434 UI/l in peak creatine kinase (from 2435 [1350–4224] UI/l to 2001 [1132–3488] UI/l, p=0.130) and of 18 ng/ml in peak troponin I (from 122 [42–204] ng/ml to 104 [51–217] ng/ml, p=0.566) were noted after initiation of the new protocol.
Excluding patients with FMC in non-PCI-capable hospitals (who did not obtain an absolute reduction in SD or total ischemia time with the new protocol), the new protocol achieved a significant reduction of 385 UI/l in peak creatine kinase (from 2227 [1355–4181] UI/l to 1842 [1082–2688] UI/l, p=0.022) and a borderline reduction of 42 ng/ml in peak troponin I (from 123 [42–221] ng/ml to 81 [46–188] ng/ml, p=0.058).
In addition, selecting only after-hours patients, who benefited most from the new protocol, significant reductions of 716 UI/l in peak creatine kinase (from 2544 [1554–5460] UI/l to 1828 [1117–2827] UI/l, p=0.009) and of 47 ng/ml in peak troponin I levels (from 137 [60–257] ng/ml to 90 [50–194] ng/ml, p=0.048) were observed with the new protocol.
DiscussionReduction of ischemia times in STEMI is a priority goal for contemporary health systems. Several regional programs with this purpose have shown promising results,8–11 and various prehospital primary PCI activation strategies (similar to that initiated in our hospital, but with smaller sample sizes) have achieved improvements in ischemia times.12,13
SD is the best ischemia time interval to evaluate a regional primary PCI network, as it involves the coordination of all of the network's components. Furthermore, it is directly related to in-hospital mortality in STEMI.5,14,15 Our new protocol achieved a 35-min reduction in CLAD and a 37-min reduction in SD. There were no significant differences between the sum of diagnosis delay plus transfer time, or in the duration of primary PCI, which implies that the observed SD reduction was mainly driven by the improvement in CLAD.
It was not the objective of our study to determine whether such improvements have prognostic consequences, but previous studies have shown that reductions of 30 min or more in SD are associated with lower in-hospital mortality.16 Furthermore, we observed a non-significant reduction in infarct size, estimated by peak creatine kinase and troponin I levels, with the new protocol. This reduction was statistically significant for the subgroups of patients who benefited most from the new protocol, i.e. after-hours patients and those with FMC in other than a non-PCI-capable hospital.
Recently, the mean system delays of different European countries during the year 2009 were published,17 ranging from 60 min in Belgium to 177 min in Serbia. The mean SD in Spain was 98 min. Another recent study in Barcelona city (Spain) observed an SD of 85 (66–110) min.11 In the USA, the median D2B time at the end of 2010 was 64 min.18
The treatment delay once patients arrive at our hospital (D2B time) was similar to that in other western countries.17,18 However, the sum of diagnosis delay and transfer time in our study was higher, which explains the observed SD differences. In a recent US registry, transfer time (without including diagnosis delay) was 35 (25–49) min for patients with out-of-hospital FMC,18 while the sum of diagnosis delay plus transfer time was 71 (60–105) min in our study after initiation of the new primary PCI protocol.
Previous studies have demonstrated that direct transfer to the catheterization laboratory when patients arrive at the hospital reduces ischemia times and improves survival.19–21 Our new primary PCI protocol was designed to achieve this goal (CLAD=0), as prehospital activation of the cardiac catheterization team may enable them to arrive at the hospital and prepare the catheterization laboratory before the patient's arrival. Concerning this objective, the percentage of patients transferred directly to the catheterization laboratory on hospital arrival rose from 28.1% before initiation of the new protocol to 44.0% afterwards.
Nevertheless, achieving CLAD=0 depends on different factors according to the time of the day. During working hours, as there is a cardiac catheterization team at the hospital, CLAD is determined by the availability of a free catheterization laboratory ready for the procedure. Prehospital activation can make this more feasible, but the actual influence on SD may be small. By contrast, after hours, CLAD depends on activation of the cardiac catheterization team plus patient transfer time, as there is virtually always a free catheterization laboratory. For this reason, the new primary PCI protocol was intended to improve treatment delays, especially after hours. The observed results support this premise.
The lack of in-hospital cardiac catheterization teams in several PCI-capable centers after hours explains the longer treatment delays observed in after-hours patients in various registries.22,23 Before initiation of the new primary PCI protocol, this was also seen in our hospital, but, importantly, the new protocol has succeeded in eliminating the differences in treatment delays between working-hours and after-hours patients.
Finally, the differences in SD between patients with various FMC locations were analyzed. In agreement with other studies,21 SD was longer in patients with FMC in non-PCI-capable hospitals, due to a longer transfer time. This is due to the distance from these non-PCI-capable hospitals to our hospital (which ranges from 16.2 km to 89.8 km), and to the lack of on-site ambulances with advanced life support: the referring hospitals must call a central ambulance coordinating center, which sends the nearest available ambulance with advanced life support to the hospital to pick up the patient. Hence, ambulances must make a time-consuming back-and-forth journey.
In a recent US study,14 patients with FMC in non-PCI-capable hospitals lost the chance of being treated with a SD ≤120 min when the traveling time between the FMC center and the PCI-capable hospital was greater than 45 min. Regional STEMI treatment programs should consider geographical and local transport criteria in order to make primary PCI the reperfusion treatment for the majority of patients, and, conversely, to define fibrinolysis as the first-choice treatment for patients with FMC far from a PCI-capable hospital.
Study limitationsThe main limitation of the study is the retrospective design of the data analysis. The data were, however, collected prospectively. Another limitation is that the study collected data from the first months after initiation of the new protocol; in the initial stages after a protocol change many problems may appear that are solved during the following months. We did not detect problems with implementation of the new protocol or prehospital activation, but they may have been unnoticed.
Moreover, the sample size was not sufficient to assess differences in clinical outcomes.
ConclusionsIn conclusion, the new primary PCI protocol, which included the prehospital activation of the cardiac catheterization team, resulted in a significant reduction of 37 min in SD. There was an absolute increase of 27.3% in the percentage of patients treated with SD ≤120 min.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.










 CLAD: cath lab activation delay;
CLAD: cath lab activation delay;  PCI protocol.
PCI protocol.  PCI protocol: after-hours patients.
PCI protocol: after-hours patients. 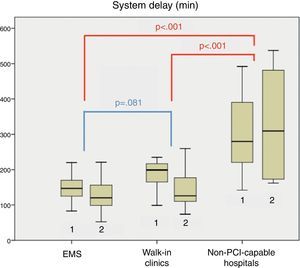 PCI protocol. 2: After initiation of the new primary
PCI protocol. 2: After initiation of the new primary 

