Non-invasive assessment of ischemic heart disease remains a challenging task, even with a large armory of diagnostic modalities. Positron emission tomography (PET) is an advanced radionuclide technique that has been available for decades. Originally used as a research tool that contributed to advances in the understanding of cardiovascular pathophysiology, it is now becoming established in clinical practice and is increasingly used in the diagnosis and risk stratification of patients with ischemic heart disease. PET myocardial perfusion imaging has a mean sensitivity and specificity of around 90% for the detection of angiographically significant coronary artery disease, and is also highly accurate for assessing the prognosis of patients with ischemic heart disease. Depending on the radiotracer used, it can provide information not only on myocardial perfusion but also on myocardial metabolism, which is essential for viability assessment. The potential of this imaging technique has been further increased with the introduction of hybrid scanners, which combine PET with computed tomography or cardiac magnetic resonance imaging, offering integrated morphological and functional information and hence comprehensive assessment of the effects of atherosclerosis on the myocardium. The scope of this review is to summarize the role of PET in ischemic heart disease.
A multiplicidade de técnicas de diagnóstico existentes, para avaliação da doença cardíaca isquémica, pode representar um desafio na escolha da mais adequada se não forem conhecidas as características de cada uma, nomeadamente, potencialidades, disponibilidade, riscos inerentes e custos. A tomografia de emissão de positrões (PET) é uma técnica de imagem com várias décadas de evolução. Usada inicialmente no campo da investigação, contribuiu para avanços significativos na compreensão da fisiopatolologia cardiovascular. Atualmente, tem um papel cada vez mais relevante no diagnóstico e estratificação de risco da doença cardíaca isquémica. A imagem de perfusão miocárdica por PET tem uma especificidade e sensibilidade médias de 90% para a deteção de doença coronária arterial significativa. Dependendo do radiofármaco escolhido, a informação poderá versar sobre a perfusão miocárdica mas também sobre o seu metabolismo, essencial, na apreciação da viabilidade. O potencial desta técnica de imagem aumentou com a introdução das câmaras híbridas que a combinam com a tomografia computorizada ou com a ressonância magnética cardíaca. Estas integram informação morfológica e funcional, fornecendo uma avaliação completa das consequências da aterosclerose no miocárdio. Com esta revisão pretendeu dar-se uma panorâmica da aplicação da PET no âmbito da doença cardíaca isquémica.
Recent guidelines recommend that patients with ischemic heart disease (IHD) have their care driven by risk assessment.1 Structural and functional information provided by different imaging techniques aids the physician in assessing different aspects of the disease. The recent shift in the management of IHD from an anatomical to a functional gold standard has highlighted the importance of functional imaging techniques.
Positron emission tomography (PET) is a nuclear medicine imaging technique that uses radiotracers to produce images of radionuclide distribution with an exterior detector system.2 These tracers can provide information on a wide range of biological pathways by non-invasive methods, using physiological substrates labeled with positron-emitting radionuclides. PET enables the assessment of flow-limiting IHD by analyzing myocardial perfusion, function and metabolism.
The role of contemporary radionuclide myocardial perfusion imaging (MPI) in the diagnosis and management of IHD is well established. Although qualitative or semi-quantitative assessment of regional perfusion is most used in clinical practice, it has limitations in determining the extent of IHD, especially in patients with multivessel disease.3,4 Another limitation of semi-quantitative perfusion assessment that is applicable to all imaging techniques is its inability to delineate the extent and severity of diffuse atherosclerosis and microvascular dysfunction.
Quantitative PET measurement of myocardial blood flow (MBF) in absolute terms (ml/g/min) potentially represents a paradigm shift in the assessment and management of patients with IHD.
Although PET is considered the current gold standard for quantitative non-invasive assessment of myocardial perfusion and viability, various factors have hindered its widespread clinical application, including limited availability of scanners and tracers and high costs.5
Positron emission tomographyPrinciples of positron emission tomography imagingLike single-photon emission computed tomography (SPECT), PET relies on external detectors to image the distribution of radiotracers with known characteristics labeled with positron-emitting isotopes. Positrons are the antimatter counterpart of electrons, and when the two interact, the electron-positron annihilation event6,7 results in two 511-keV gamma photons being emitted simultaneously at approximately 180° to each other that are sensed by a detector ring.8 Coincidence detection without the need for physical collimators is one of the advantages of PET over SPECT. Although SPECT remains the most widely employed modality in most centers with nuclear cardiac imaging, it has important limitations that PET can overcome. Table 1 summarizes the characteristics of PET and SPECT.
Characteristics of single-photon emission computed tomography and positron emission tomography.
| Cardiac PET | Cardiac SPECT | |
|---|---|---|
| Availability | Limited | Widespread |
| Photon detection | Coincidence detection with crystals positioned 360° around the patient | Detection of single photonsDual-headed gamma cameras that rotate around the patient (most common); novel multi-headed solid-state cameras positioned 180° around the patient |
| Collimation | None | Required |
| Attenuation correction | More accurate | Less accurate |
| Spatial resolution | 4-7 mm | 10-15 mm |
| Protocol | <1 hour | Up to 5hours on 1 or 2 days |
| Radiation exposure | <5 mSv | 7-10 mSv |
| Myocardial perfusion images | Absolute quantification possible | Usually semi-quantitative |
| Hybrid with CT | Yes | Yes |
| Hybrid with MRI | Yes | No |
CT: computed tomography; MRI: magnetic resonance imaging; PET: positron emission tomography; SPECT: single-photon emission computed tomography.
PET imaging reflects cardiac physiology rather than anatomy. The information obtained depends on the radioactive nuclides used and their imaging characteristics. Therefore, selection of the nuclide depends on multiple factors besides the aim of the PET imaging program. Table 2 compares the characteristics of the different tracers used.
Characteristics of tracers used in positron emission tomography.
| Myocardial perfusion tracers | Myocardial metabolism tracers | ||||
|---|---|---|---|---|---|
| 13NH3 | 82Rb | H215O | 18F flurpiridaz | FDG | |
| Production method | Cyclotron | Generator | Cyclotron | Cyclotron | Cyclotron |
| On-site cyclotron | Required | Not required | Required | Not required | Not required |
| Half-life | 9.97 min | 76 s | 123 s | 110 min | 119 min |
| Kinetics | Metabolically trapped in myocardium | Freely diffusible, metabolically inert | Metabolically trapped in myocardium | Metabolically trapped in myocardium | Metabolically trapped in myocardium |
| Scan duration | 20 min | 6 min | 6 min | 20 min | 10-30 min |
| Gating /LV function | + | + | - | + | X |
| Radiation dose | ∼1 mSv | ∼3 mSv | ∼ 0.4 mSv | ∼ 4 mSv | X |
| Quantification | Good | Moderate | Excellent | Very good | X |
13NH3: 13N-labeled ammonia; FDG: 18F-2-fluoro-2-deoxyglucose; 82Rb: rubidium-82; H215O: O15-labeled water; LV: left ventricular.
Four radiotracers are mainly used for myocardial perfusion imaging: 15O-labeled water (H215O), 13N-labeled ammonia (13NH3), rubidium-82 (82Rb), and an 18F-labeled PET perfusion tracer, 18F-BMS-747158-02 (flurpiridaz).9 Each has specific properties that make one preferable over another in individual situations.
Myocardial metabolism imagingUnder normal conditions, oxidative phosphorylation is the principal pathway involved in energy production in cells. In a normal heart, the major source of adenosine triphosphate (ATP) is oxidation of free fatty acids, rather than of carbohydrate.7 During ischemia, reversible metabolic adaptation will occur to enable myocytes to survive in a low-oxygen environment. Mitochondrial oxidation is suppressed and anaerobic metabolism can proceed. Under these circumstances, exogenous glucose uptake and glycogen breakdown are increased, glycolysis is stimulated, and ATP can be produced from the anaerobic catabolism of glucose with concomitant formation of lactate.10
In view of the myocardium's use of exogenous glucose, PET imaging uses 18F-2-fluoro-2-deoxyglucose (FDG) to trace glucose uptake. FDG is a glucose analogue that is transported into the myocyte by the same trans-sarcolemmal carriers (GLUT-1 and GLUT-4) as glucose and phosphorylated to FDG-6-phosphate by hexokinase.9 It is not further metabolized or used in glycogen synthesis or aerobic glycolysis. Because dephosphorylation and return of the radiotracer to the blood is minimal, it becomes metabolically trapped in the myocardium, permitting PET imaging of regional glucose uptake that reflects overall cell glucose uptake. FDG uptake may be increased in hibernating but viable myocardium; uptake in regions with reduced blood flow at rest has become a marker of hibernation.11 FDG is produced by a cyclotron and decays with the emission of a positron with a half-life of 110min. The 110-min half-life of FDG gives sufficient time for synthesis and purification, with commercial distribution within a radius of several hours’ travel from the production site.8
Nevertheless, the diagnostic quality of FDG imaging is critically dependent on hormonal milieu and substrate availability. Images with FDG depend on patient preparation, as described in the imaging guidelines for PET.8
Positron emission tomography in the assessment of myocardial perfusion and viabilityPerfusion assessmentThe clinical scenario and the capabilities of the technique influence the choice of cardiac imaging test. The following sections discuss the use of cardiac PET in the assessment of IHD at different stages and clinical presentations.
Interpretation of perfusion dataSimilarly to SPECT, in clinical practice PET perfusion images are most commonly graded visually in a qualitative manner. Qualitative defect estimation should be performed by describing the location of the abnormal segments involved and their extent in the left ventricle. The extent of the defect may also be qualitatively described as small (5-10% of the left ventricle), medium (10-20%), or large (>20%).8 Defect severity is typically expressed qualitatively as mild, moderate or severe depending on its similarity to background tracer activity.
Stress and rest myocardial perfusion image sets are compared in order to determine the presence, extent and severity of stress-induced perfusion defects and to determine whether such defects represent regions of myocardial ischemia or infarction. Regions with only stress-induced defects represent ischemia. Perfusion abnormalities on stress images which remain unchanged on rest images are termed fixed defects and most often represent areas of prior myocardial infarction (MI). Areas of partial reversibility represent the presence of both scar and ischemia (Figure 1).
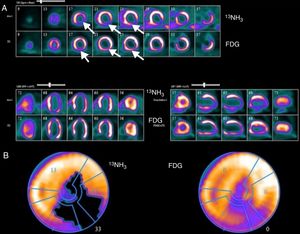
Example of assessment of myocardial viability with positron emission tomography imaging. (A) Top rows: 13N-labeled ammonia (13NH3) is used as a tracer of myocardial blood flow at rest in short-axis images starting at the apex and moving toward the base of the heart (upper image), horizontal long axis (lower left) and vertical long axis (lower right). Myocardial perfusion is markedly decreased in the apical, inferior and inferolateral regions (white arrows); Bottom rows: 18F-2-fluoro-2-deoxyglucose (FDG) is used as a tracer of myocardial glucose metabolism. FDG uptake is enhanced relative to blood flow, demonstrating a pattern of perfusion-metabolism mismatch (white arrows) in the abnormally perfused myocardial regions, indicative of viable or hibernating myocardium; (B) polar map of viability study. The left polar map plot displays the extent of the rest perfusion defect (black area); the right polar map plot shows FDG uptake in the rest perfusion defect area indicating metabolic viability. Image source: Instituto das Ciências Nucleares Aplicadas à Saúde (ICNAS).
In addition to qualitative and semi-quantitative grading, PET also enables absolute quantification of perfusion. Quantitative blood flow approaches offer an objective interpretation that is inherently more reproducible than visual analysis. Blood flow can be assessed globally and regionally. When epicardial coronary arteries are narrowed by atherosclerotic disease, coronary autoregulation attempts to normalize MBF by reducing the resistance of distal perfusion beds at the arteriolar level, thus maintaining myocardial oxygen supply.12 MBF can be estimated using various techniques, including coronary catheterization with a Doppler flow wire, but all of these techniques are invasive and thus have limitations for clinical practice.13,14 PET has become the non-invasive imaging modality of choice for the quantification of MBF.
Resting MBF is the absolute quantity of blood that the myocardium receives per minute per gram of tissue under baseline conditions. MBF conceptually refers to the measurement not just of epicardial flow, but also microvascular flow and function.15 MBF values in normal individuals at rest range between 0.6 and 1.3ml/min/g (mean 0.98±0.23ml/min/g).16,17
Hyperemic MBF represents the maximum blood flow that can be supplied to the heart during maximum vasodilation of the coronary vascular bed. This is usually achieved through pharmacologically induced stress.18
Myocardial flow reserve (MFR) is the ratio of stress to resting MBF. MBF and MFR values obtained by PET are not the same as those from invasive measurement of epicardial coronary flow reserve (CFR) or fractional flow reserve (FFR), although they are closely linked.6,19 MFR, unlike FFR, assesses the combined effects of stenosis and microcirculation, but cannot differentiate the effects of either independently. It is thus possible to have discordant MFR and FFR values in the case of a dominant focal lesion with minimal microcirculatory disease, or when there is diffuse epicardial disease combined with severe microcirculatory disease.19,20
There is currently limited information on the optimal threshold to distinguish pathological from normal hyperemic MBF and MFR.21 It is accepted that MFR values can be interpreted as follows:
MFR >2.3 indicates low risk (assuming that there is no lower regional value)8,18;
MFR <1.5 suggests significantly decreased flow reserve (in the absence of concomitant increased resting MBF), and is associated with increased cardiac risk.8,18
These values were recently confirmed by a multicenter study that established an optimal threshold of 2.3ml/min/g for hyperemic MBF and 2.5 for MFR compared to invasive FFR measurements.22 Alternatively, MBF values can be interpreted on a continuous scale for diagnostic and prognostic purposes as well as for subsequent clinical decision-making.21
It is important to assess both hyperemic MBF and MFR in all subjects. In addition, like most other imaging parameters, MBF should be considered as supplementary, in conjunction with other clinical characteristics and image findings, when used for diagnosis or to guide patient management. Table 3 summarizes the potential applications of absolute flow quantification.
Potential applications of absolute flow quantification.
| Application | Description |
|---|---|
| Early atherosclerosis | Assessment of microvascular dysfunction in diabetes, hypertension, metabolic syndrome, etc. |
| Advanced atherosclerosis | Improved detection of multivessel disease |
| Evaluation of hemodynamic significance of stenosis | |
| Non-atherosclerotic microvascular disease | Stable angina or ACS with normal coronary angiogram |
| Transplant vasculopathy | |
| Dilated cardiomyopathy | |
| Hypertrophic myopathy | |
| Determination of prognosis | |
| Evaluation of therapies | |
In conclusion, absolute quantification of myocardial blood flow expands the scope of conventional relative MPI from identifying only end-stage epicardial IHD to the earlier identification and characterization of abnormalities in coronary endothelial function and subclinical stages of IHD (microvascular dysfunction).15
Diagnostic accuracy of positron emission tomography in ischemic heart diseaseThe majority of studies exploring the diagnostic accuracy of PET perfusion imaging for detection of IHD have been conducted with static uptake of 82Rb and 13NH3.21 Compared with SPECT, perfusion imaging using PET consistently yields higher diagnostic accuracy.23-25 An early review based on a pooled analysis of 79 studies with SPECT of nearly 9000 patients reported a mean sensitivity of 86% and mean specificity of 74% for detecting >50% angiographic stenosis, with improved specificity using attenuation correction methods.26 With PET perfusion imaging, the reported mean sensitivity reached 90% and the mean specificity 89% for detecting >50% angiographic stenosis, as derived from a pooled analysis of nine studies of almost 900 patients.27 Although many of these studies had important limitations (most were small retrospective series, using older two-dimensional PET systems, and most did not quantify MBF), more recent data support the same conclusions.28,29 Two recent meta-analyses demonstrated that PET MPI is superior to SPECT MPI.25,30 Sensitivity and specificity for PET in these meta-analyses ranged from 90% to 93% and 81% to 88%, respectively.
Results of the Prospective compArison of CardIac PET/CT, SPECT/CT perFusion imaging and CT coronary angiography with Invasive Coronary angiography (PACIFIC) trial were published in 2017.31 This was the first head-to-head comparison of the most commonly used non-invasive techniques against FFR. All modalities were compared and the investigators found that PET was more accurate (85%) than cardiac computed tomography angiography and SPECT for diagnosing coronary ischemia. Although the diagnostic accuracy of PET imaging was similar to that reported in previous studies, this was the first head-to-head comparison of anatomic and functional imaging techniques in suspected IHD.
Measurement by PET of myocardial perfusion in absolute units further improves its diagnostic accuracy. Testing has been less extensive for quantitative perfusion imaging; however, there is growing evidence of its superiority over static uptake image grading.32–35 Patients with multivessel disease (i.e. balanced ischemia), early stage blood flow impairment or microvascular disease, and those with high body mass index could benefit most from this quantitative assessment.21,33
Although its cost-effectiveness in high-throughput centers has been demonstrated, the clinical utility of PET is still constrained by high upfront cost and low availability compared with SPECT.36
Prognostic value of positron emission tomography in ischemic heart diseasePET MPI has been shown to have prognostic value in the context of both normal and abnormal scans, providing incremental risk information in patients with known or suspected IHD. The extent and severity of PET-derived perfusion defects have also been shown to provide valuable prognostic information beyond traditional cardiovascular risk factors.37–39 A normal scan indicates low risk (<1% annual cardiovascular [CV] event rate) while an abnormal scan indicates a worse prognosis (>4.2% annual event rate), the risk increasing with the extent of ischemia and the severity of the findings.37–39 Furthermore, the integration of perfusion and functional imaging enables assessment of rest left ventricular (LV) ejection fraction (LVEF), stress LVEF and LVEF reserve (stress LVEF - rest LVEF) as well as LV volumes, providing incremental prognostic value.40,41 Even in the presence of angiographically significant IHD, normal findings on stress MPI are generally associated with a low risk of CV events (around 1% per year).42
As well as LVEF, LVEF reserve is also a marker of extensive anatomic obstructive IHD (providing higher sensitivity than perfusion data alone, 50% vs. 79%) and an independent and incremental marker for patient outcome.43 Even after accounting for differences in clinical factors and perfusion findings, patients with LVEF reserve <0 have a higher annual risk of coronary events (2.1% vs. 5.3%, p<0.001) and all-cause death (4.3% vs. 9.2%, p<0.001) compared to patients with LVEF reserve >0.39,41,44
Nevertheless, the risk of cardiac events is an individual analysis that should consider all factors besides imaging information. Specific groups, such as the elderly and those with diabetes or known IHD, have a somewhat higher annual event rate (1.4-1.8%) despite normal MPI. The warranty period of a normal PET MPI in the setting of IHD is around two years, depending on risk factor control.45
Several studies have also documented the greater prognostic value of PET-derived MFR compared to clinical factors and perfusion defect size and severity in patients with known or suspected IHD.46,47 The addition of MFR, as measured by PET, led to correct reclassification of estimated risk categories in 35% of patients with previously intermediate risk of death.48 Although global MFR is only modestly associated with the overall extent and severity of angiographic disease, both low MFR and severe angiographic disease were independently associated with adverse clinical events.48 Global MFR is associated with major CV events independently of luminal angiographic severity and modifies the effect of coronary revascularization, underscoring the morbidity associated with diffuse atherosclerosis and microvascular disease.49
Given that it indirectly reflects microvascular disease, MFR also has prognostic value in patients with diabetes and with chronic kidney disease.50
The combination of these findings in prognostic assessment supports the conclusion that overall atherosclerotic disease burden and resultant macro- and microvascular ischemia, with or without obstructive epicardial lesions, are important contributors to overall CV risk. As such, PET-derived MFR may have particular prognostic importance as a sensitive global biomarker for functional IHD, especially in its ability to integrate complex pathophysiological sequelae at the target organ of interest.49 Although the role of MFR in establishing prognosis is becoming clearer, more data are needed to generate sufficient evidence of its value.
Assessment of myocardial viabilityThere are numerous radiotracers that can measure cellular glucose metabolism either directly, such as FDG and 11C-glucose, or indirectly, such as 11C-palmitate and the various 18F-labeled fatty acid analogues.8 This discussion will focus on FDG.
Clinical setting and the role of glucose metabolism assessmentThree pathophysiological phenomena have been described in the setting of IHD in which myocardium is viable but dysfunctional: stunning, hibernation and remodeling.
Stunning is a state of transient regional contractile impairment, usually resulting from an ischemic insult, which persists for hours or weeks even after restoration of coronary flow (i.e. post-ischemic dysfunction). Recovery of myocardial function is spontaneous provided that myocardial perfusion remains normal.43 The duration of stunning is directly proportional to the duration of the preceding ischemia.51
Hibernation refers to dysfunctional myocardium that is in a state of metabolic downregulation in response to chronic or repetitive ischemia. However, resting flow in hibernating myocardium may not be decreased to the extent that would account for the degree of cardiac dysfunction. In most cases, the impairment is only detected through reduced MFR, with reduced rest MBF only being seen in the most advanced cases. In this way, hibernation may represent a spectrum, with chronic repetitive stunning showing normal or near normal resting perfusion and impaired MFR at one end and reduced rest MBF at the other. Recovery of function in hibernating myocardium also requires coronary revascularization to restore adequate MFR.43
Lastly, remodeling can occur, resulting in dysfunctional myocardium adjacent to the infarct or hibernation core that may or may not improve with revascularization, depending on improvement in other regions of the ventricle.51
In the setting of diminished, but not absent, regional MBF, reversible metabolic changes will occur, as an adaptive measure to sustain myocardial viability. When MBF is absent, irreversible metabolic changes occur, followed by MI and cell death.8 Consequently, demonstration of preserved glucose metabolism by FDG is a marker of myocardial viability and the detection of viable myocardium is accurately estimated by referencing the level of myocardial glucose metabolism to the level of MBF.
Image interpretation and integrationUsing a sequential perfusion-metabolism approach gives the most complete information, yielding different interpretation possibilities.
Normal perfusion images effectively guarantee myocardial viability, and therefore under conditions of normal perfusion it may not be necessary to continue with metabolic imaging. However, a pattern of normal perfusion coupled with reduced FDG uptake (so-called reversed perfusion-FDG mismatch) has been described in patients with left bundle branch block52 and also under conditions of repetitive myocardial stunning (e.g. early post-MI revascularization and in diabetic patients).43,53 This most likely reflects regions of jeopardized but viable myocardium, as the perfusion tracers reflect active metabolic trapping (Na+-K+ ATPase system for 82Rb and 13N-ammonia).8
In regions of reduced MBF, an increase in myocardial metabolism by one or more grade therefore reflects a perfusion-metabolism mismatch, hence myocardial viability. By contrast, a regional reduction in FDG uptake in proportion to regional reduction in myocardial perfusion reflects the presence of a perfusion-metabolism match, hence myocardial scar or non-viable tissue.
Prognostic assessment and contribution to patient managementObservational evidence suggests that FDG PET, as a viability imaging tool, has the greatest sensitivity for predicting global LV functional recovery following revascularization, compared with SPECT, dobutamine stress echocardiography (DSE) and cardiac magnetic resonance imaging (MRI) (p<0.05 vs. other modalities).54–56 Studies have consistently showed radionuclide techniques to be more sensitive for prediction of functional recovery, whereas techniques challenging contractile reserve such as cardiac MRI and DSE are more specific.56
The degree of scarring on FDG PET has also been shown to be an important predictor of improvement in LVEF following revascularization. Beanland et al. found that the change in LVEF after revascularization was significantly greater in patients with less scar tissue (change of 9.0%, 3.7%, and 1.3% for small, moderate, or large scars, respectively).57 Compared to scar, dysfunctional myocardium classified as hibernating or stunned by PET has a high chance of functional improvement following revascularization.51 There is evidence from multiple, predominantly retrospective, observational studies that the presence of hibernating myocardium involving as little as 5-7% of the left ventricle is associated with an outcome benefit from revascularization.58,59
However, although there is a wealth of observational evidence showing the benefit of revascularization in terms of viability,59–61 the subject remains controversial after the primary results of the two largest prospective studies involving viability imaging, the Surgical Treatment for Ischemic Heart Failure (STICH) viability substudy62 and PET and Recovery Following Revascularization (PARR-2),63 which did not yield clear and conclusive positive findings concerning an imaging-guided approach to revascularization.
The PARR-2 trial assessed whether the use of FDG PET in clinical decision-making leads to better clinical outcomes compared with standard care where FDG PET was not available. The study population included patients with LVEF ≤35% who were being considered for revascularization, transplantation or heart failure work-up. The primary outcome was a composite of cardiac death, MI, or recurrent hospital stay for cardiac cause at one year. In the PET arm, the extent and severity of scar and mismatch were integrated with clinical parameters in a previously derived model for prediction of LV recovery after revascularization.63 The trial did not demonstrate significant differences between the two groups. However, in a post-hoc analysis focusing on adherence to PET recommendation, there was a significant decrease in the hazard ratio for the primary outcome compared with standard care for revascularized patients with a mismatch of at least 7%.58
In the viability substudy of the STICH trial, there was no relationship between myocardial viability and outcome benefit from revascularization.51 However, there are several limitations of this substudy that should be taken into consideration before drawing firm conclusions. PARR-2 and its substudies provided good evidence for using FDG PET to identify high-risk patients who may benefit from revascularization.58,63,64
In spite of the above results, viability imaging appears to have a role as an adjunct to decision-making for complex patients (those with previous revascularization or multiple comorbidities), in whom both the risks and potential benefits of revascularization are highest.65 At the same time, the STICH trial and its viability substudy, despite its limitations, have fueled the debate over the usefulness and appropriateness of viability imaging in patients with IHD and LV dysfunction. After the publication of this substudy the medical community eagerly awaits the results of the ongoing IMAGE-HF trial, which aims to address the role of cardiac imaging in management decisions and to ascertain which methods are most suitable according to different clinical scenarios.66
Final remarksThe research and clinical community consider that individualizing therapy is essential in order to effectively improve patient outcomes. Cardiac imaging modalities are expected to play an important role in the assessment of the individual patient's pathological condition, helping to guide the treatment of cardiovascular disease.
Cardiac PET imaging is a powerful and accurate tool for the diagnosis of IHD. Compared to SPECT, it has a wider availability of perfusion tracers that can be adapted to different patients and situations, and involves lower radiation exposure. Its prognostic value is clearly established and it is already being used to guide clinical decisions. The addition of MBF quantification yields incremental diagnostic value and prognostic information, while solving some of the interpretation issues associated with relative imaging methods such as SPECT and PET MPI. As it is able to identify various patient types, from those with risk factors and early diffuse IHD to those with advanced three-vessel disease, it can also be used to assess improvements following treatment such as lifestyle modification, exercise, optimal medical therapy and revascularization. Furthermore, MFR with PET is another sensitive tool that can reveal the presence of IHD and further improve risk stratification.
Several modalities are also now available for imaging viable myocardium, with FDG PET as the currently accepted gold standard. The concepts of hibernating, stunned and viable myocardium remain at the forefront of the debate regarding the appropriateness of revascularization for patients with IHD and significant LV dysfunction. In general, viability imaging may provide additional information for decision-making in complex patients in whom the potential risk of revascularization is greater.
There are many developments on the horizon for cardiac PET in the coming years, with new scanner technology as well as new radionuclides providing a myriad of potential applications for directly aiding patient management as well as improving understanding of multiple cardiac conditions. Although these developments are currently outpacing research that demonstrates their efficacy, it is reasonable to expect that cardiac PET will play an increasingly important role in the future.
PET imaging in its various forms will certainly help to establish a more direct relationship between an individual's diagnosis and therapy. Even so, improved standardization is needed and more research is required to determine its full impact on decisions that affect patient outcomes and resource use, and thus enable the full use of this valuable tool for diagnosis and risk stratification of patients with IHD, whatever their clinical scenario and stage of IHD. Although its clinical availability in Portugal is currently somewhat limited, the ever-increasing use of PET technology in cardiology and other fields, combined with advances in radiotracer technology, is expected to lead to an increasingly important role for cardiac PET imaging in the future.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.