Patent ductus arteriosus, a persistent communication between the descending thoracic aorta and the pulmonary artery, is one of the most common congenital heart defects. Transcatheter occlusion is an effective alternative to surgery and is currently standard of care for most patients. The authors present the results from a single center after twelve years of experience using this technique.
MethodsRetrospective analysis of medical records from all patients referred to a tertiary center for percutaneous ductus closure between January 2006 and September 2018.
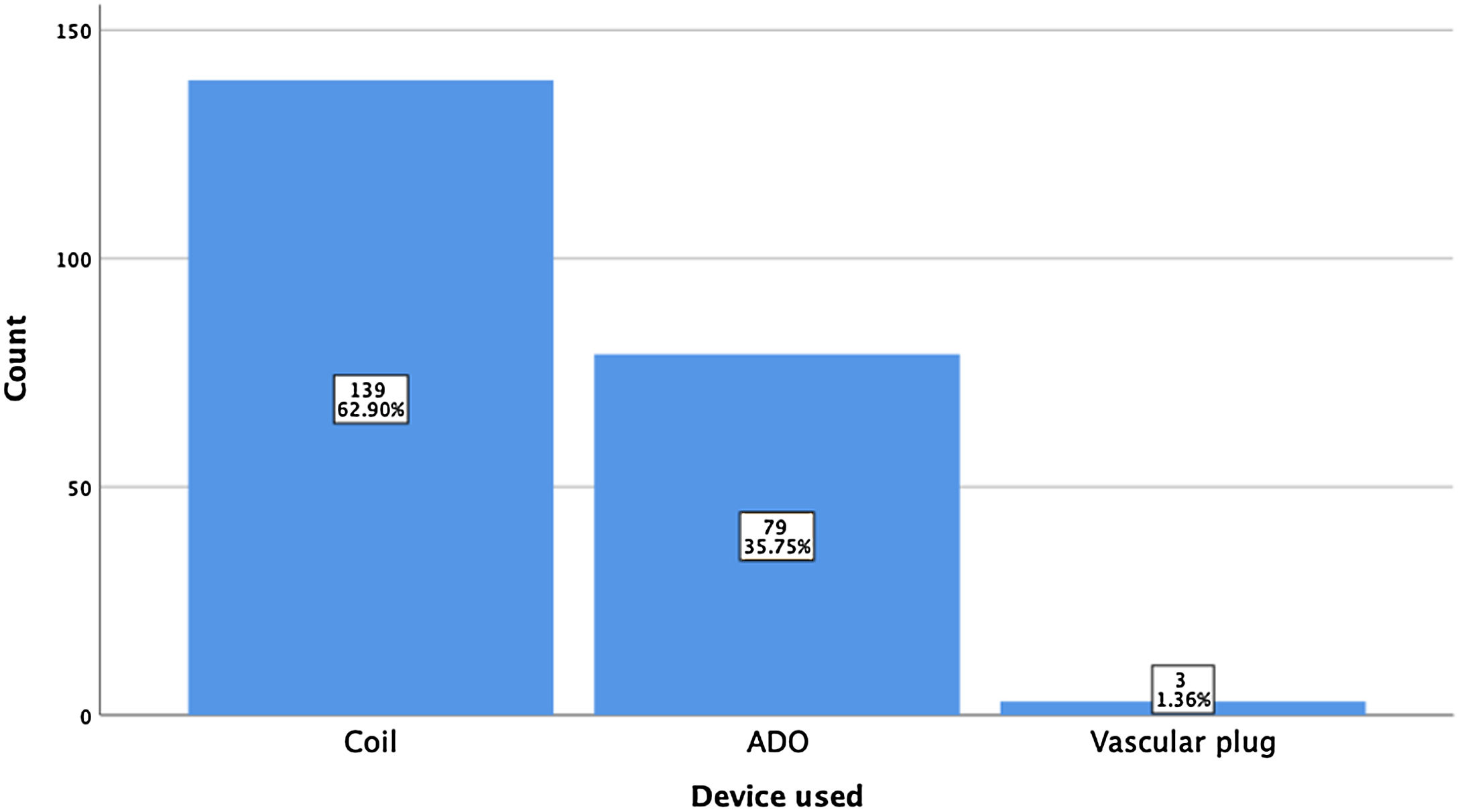
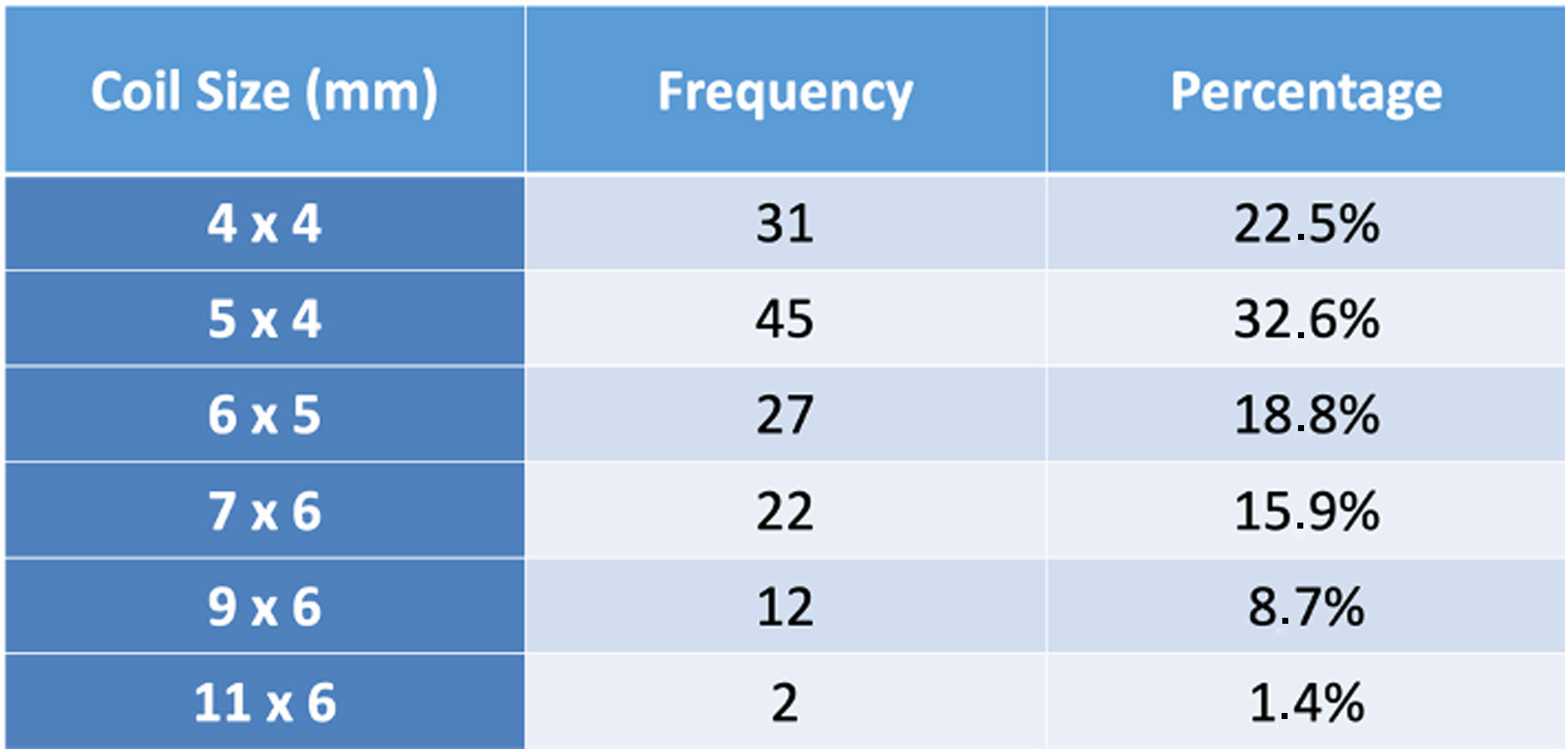
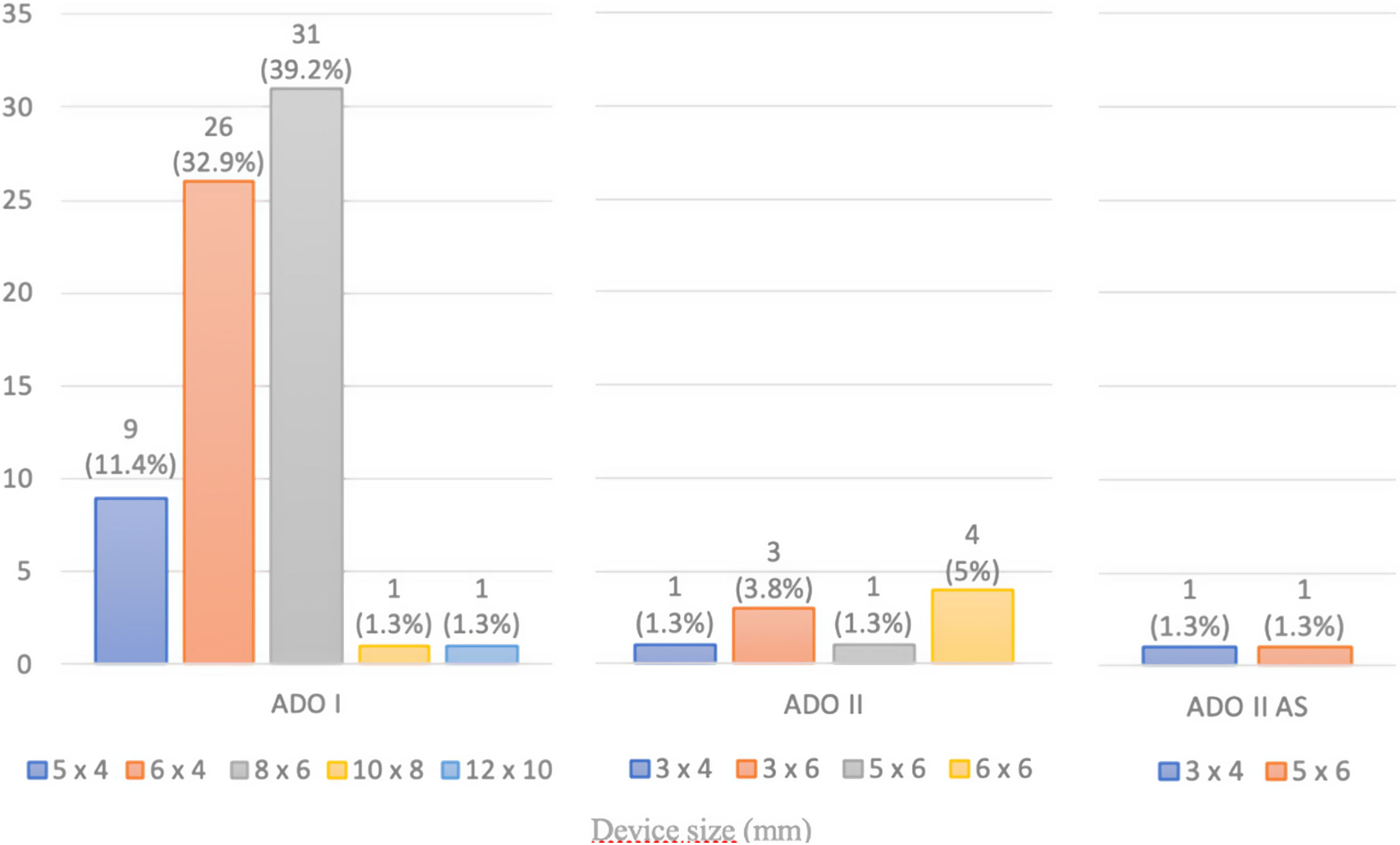
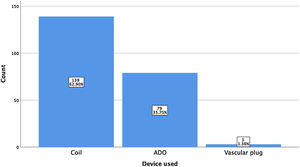
ResultsA total of 221 patients were referred, with a mean age of 5.5 years-old (16 patients were infants, with the youngest aged four months). A Nit-Occlud® coil was used 139 times (62.9%), an Amplatzer™ duct occluder 79 times (35.7%), and vascular plugs were used three times. Percutaneous closure was achieved in every treated patient, with 1.4% maintaining residual shunting. Although higher overall coil device implantation was noted, duct occluder usage has been greater since 2011. Of all the coils, 55% were either 4x4 or 5x4 mm, and 73% of all Amplatzer duct occluders were either 6x4 or 8x6 mm, which correlates to the majority of patients having a small to moderately sized ductus. No complications were noted during the procedure, with a 1.8% post-procedure complication rate (one device embolization after 48 hours and three cases of loss of arterial pulse).
ConclusionsPercutaneous patent ductus arteriosus closure was safe and effective in this setting, with a low global complication rate and similar outcomes to most equivalent centers.
A persistência de canal arterial, uma comunicação entre a aorta descendente torácica e a artéria pulmonar, é uma das cardiopatias congénitas mais comuns. O encerramento percutâneo, uma alternativa eficaz à cirurgia, corresponde atualmente ao tratamento de eleição na maioria dos doentes. Os autores apresentam os resultados de um centro de referência após doze anos de experiência com esta técnica.
MétodosAnálise retrospetiva dos registos eletrónicos de todos os doentes orientados para encerramento percutâneo num centro de referência entre janeiro de 2006 e setembro de 2018.
ResultadosForam referenciados 221 doentes, com média de 5,5 anos (16 lactentes, o mais novo com quatro meses de idade). Globalmente, 139 pacientes foram tratados com coil Nit-Occlud® (62.9%), 79 pacientes foram tratados com Amplatzer duct occluders (35,7%) e plugs vasculares foram utilizados três vezes. O encerramento percutâneo foi conseguido em todos os doentes tratados, com shunt residual em 1,4% dos casos. De todos os coils, 55% eram 4x4 ou 5x4 mm e 73% de todos os Amplatzer duct occluders eram 6x4 mm ou 8x6 mm, refletindo o facto de a maioria dos doentes apresentar canais arteriais de pequena-média dimensão. Não foram descritas complicações durante o procedimento, reportando-se uma taxa de complicações pós-procedimento de 1,8% (uma embolização de dispositivo após 48 horas e três casos de perda de pulso arterial).
ConclusãoO encerramento percutâneo da persistência de canal arterial demonstrou ser seguro e eficaz neste contexto, com uma baixa taxa de complicações e resultados semelhantes a outros centros equivalentes.
Patent ductus arteriosus (PDA) is a common congenital heart defect, with an estimated incidence of 0.05% of all live births.1 This disorder is usually identified in childhood, but may remain unrecognized until later in life. Transcatheter PDA closure was first described in 19672 and has become the standard method of treatment for most patients. Coil occlusion, first introduced in 1992, was the most common closure technique for small-sized ductus arteriosus for a long time,3 with most moderate- to large-sized ductus being closed using Amplatzer duct occluders (ADO, Abbott, USA).4 Currently, with the advent of new devices suitable for the percutaneous treatment of PDA even in low-weight preterm infants,5 the trend is towards a progressive replacement of coil implantation in small-sized lesions. Severe complications are globally rare, with device embolization being the most common reported adverse event. Altogether, various studies have concluded that short- and intermediate-term outcomes for transcatheter PDA closure are excellent,6–9 while there is only a limited number of studies reporting long-term follow-up of these patients.10–13 The authors objective was to review the results of 12 years of experience with this technique in a tertiary center, emphasizing the devices used, complications and outcomes.
MethodsAll consecutive pediatric patients referred to our center for percutaneous PDA closure from January 2006 until September 2018 were included in this study. Medical record data was collected and reviewed retrospectively. Informed written consent was obtained from the parents or legal guardians. Indications for closure were presence of cardiac murmur, left-sided volume overload detected by non-invasive cardiac imaging, or signs of heart failure. All procedures were performed under general anesthesia. Arterial access was obtained in all patients. An intravenous bolus injection of 100 IU/kg heparin was administered at the start of the procedure. Our endocarditis prophylaxis protocol consisted of cephazolin 25-30 mg/kg every eight hours for 24 hours, and no anti-aggregation was advocated. In every patient, an aortogram was performed in the lateral projection to define the morphology and size of the duct. According to these results, feasibility for percutaneous closure was determined and, when deemed feasible, appropriate devices were selected. At our department, the Nit-Occlud® occlusion device (pfm, Cologne, Germany) has been available since 2006, with the Amplatzer™ duct occluder being implemented in 2011. As standard of care, all devices were preferably deployed anterogradely. A post-implantation aortogram was obtained to check for residual shunts or any anomaly in the device position. All patients were assessed six hours after the procedure for hemodynamic instability and unpalpable distal arterial pulses. The following day, before discharge, the location of the device was noted on a chest radiograph and a transthoracic echocardiography was performed to exclude residual leakage or other complications. Clinical and echocardiographic follow-up assessments were performed at one, three, six and twelve months after the procedure, and annually thereafter.
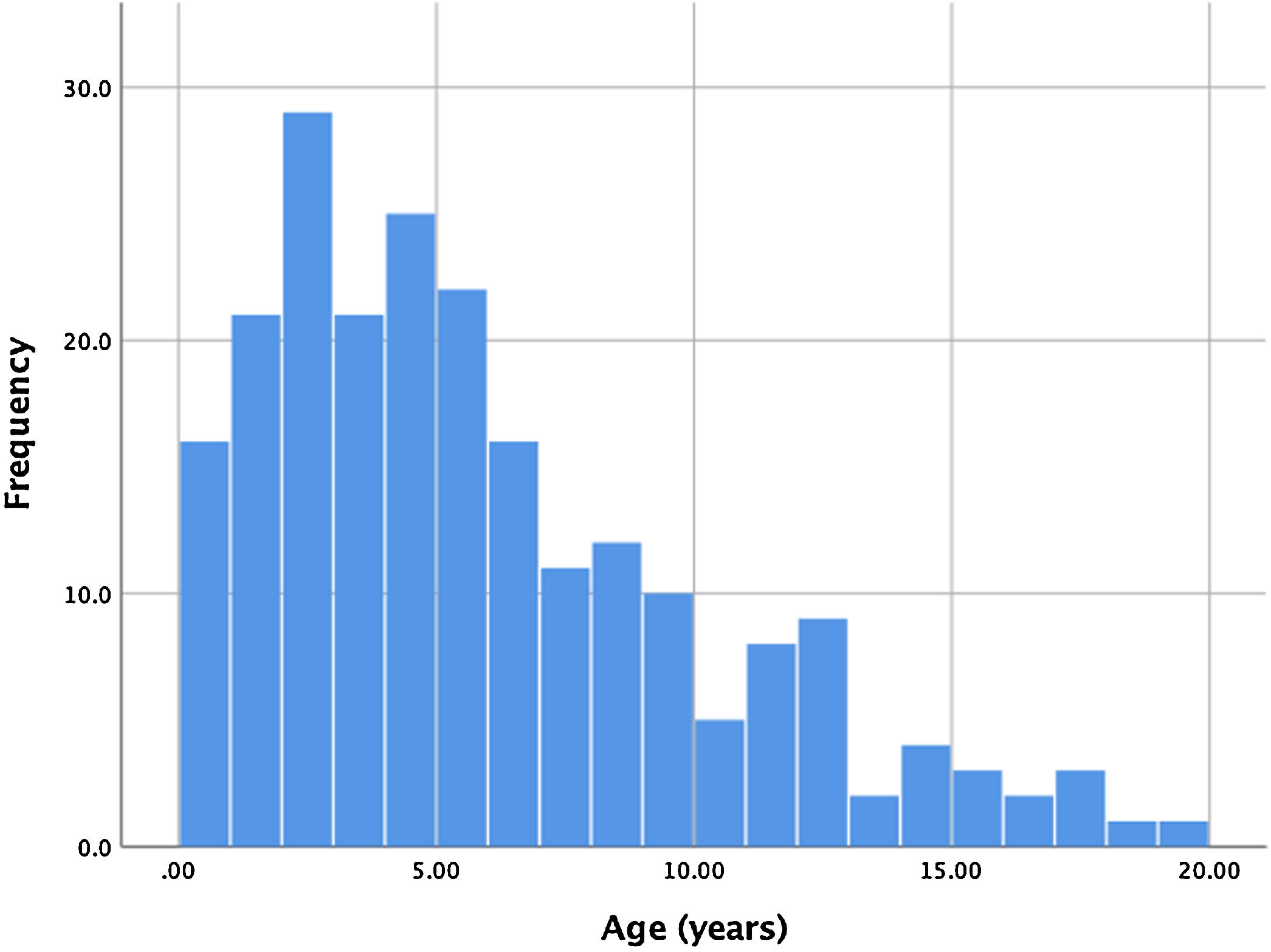
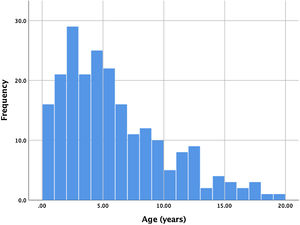
ResultsBetween January 2006 and September 2018, 221 consecutive patients were referred for percutaneous PDA closure at our center. Two patients had a history of previous ductus closure at a different institution (one percutaneous and the other surgical), presenting however a significant shunt that warranted a new procedure. Ages ranged from four months to 19 years, with a mean age of 5.6 years. Of all patients, 17 (7.7%) were infants, with the majority of patients ranging between one and seven years old. The age distribution is shown in Figure 1. Global average follow-up was 49 months (minimum one month, maximum 12 years).
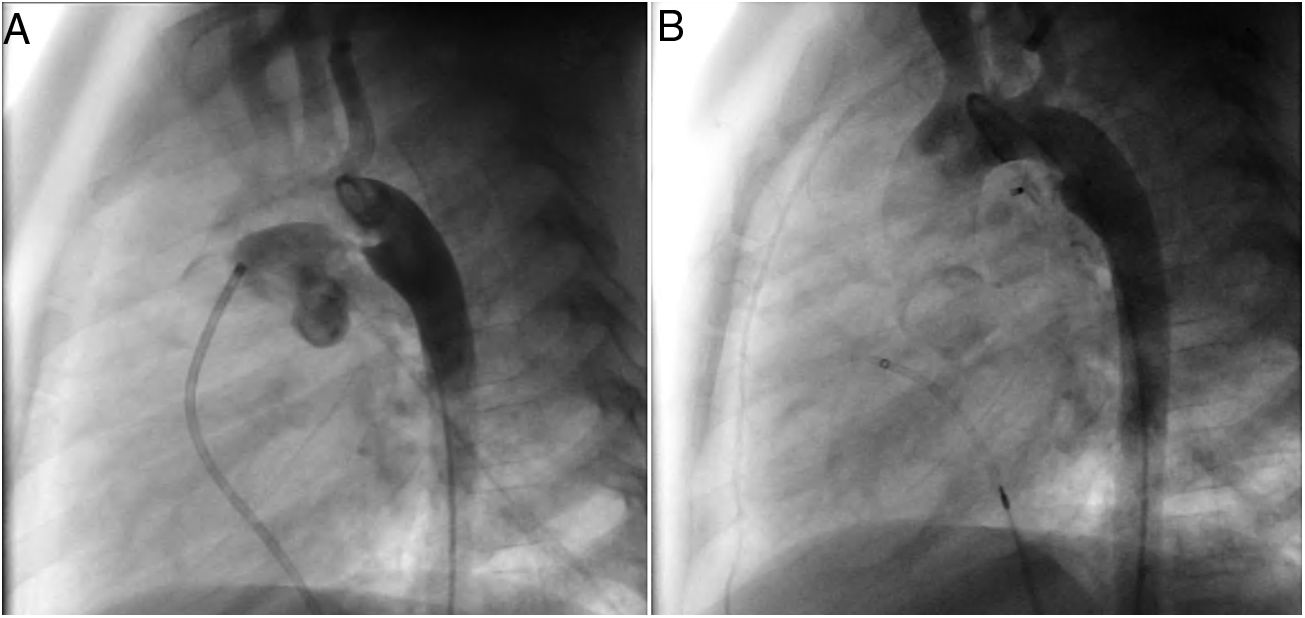
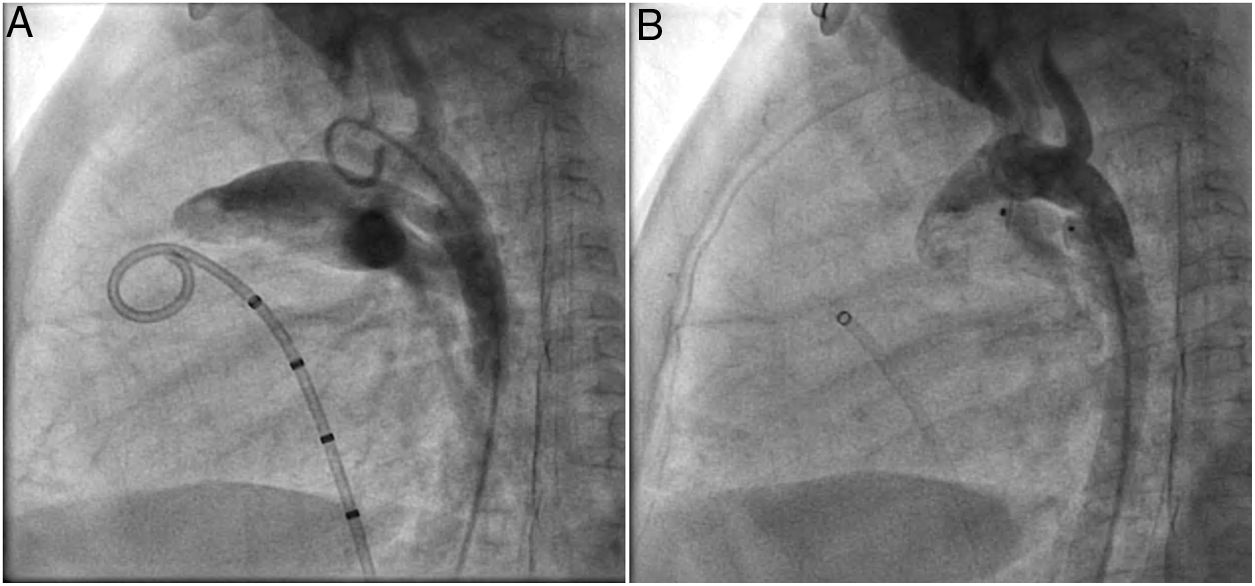
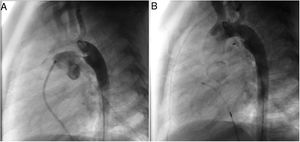
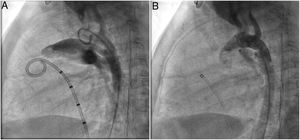
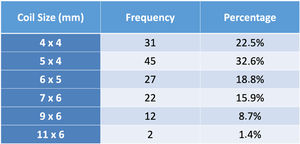
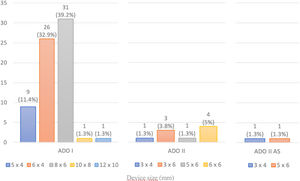
A coil was used in 139 patients (62.9%) and an Amplatzer duct occluder was used in 79 patients (35.7%). In the three remaining patients (1.4%), an Amplatzer vascular plug was used for closure (Figures 2-8). Despite the higher overall rate of coil device implantation, Amplatzer duct occluder usage had been clearly superior since 2011. Of all coil devices used, 55% were either 4x4 mm or 5x4 mm in size (Figure 9), and of all duct occluder devices used, 72% were Amplatzer duct occluder I 6x4 mm or 8x6 mm in size (Figure 10), which correlates to the fact that most patients who were referred had small- to moderate-sized ductus arteriosus. While ADO II and ADO II AS devices can be implanted in a retrograde fashion, only on two occasions was a retrograde deployment performed (both ADO II devices).
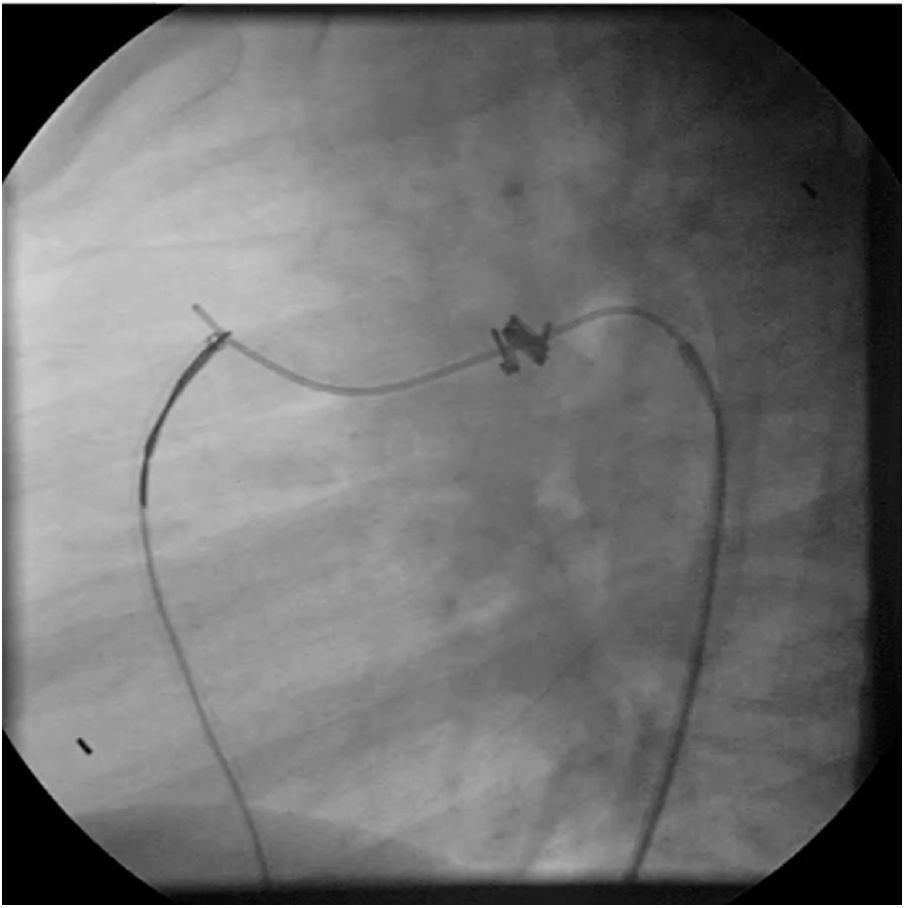
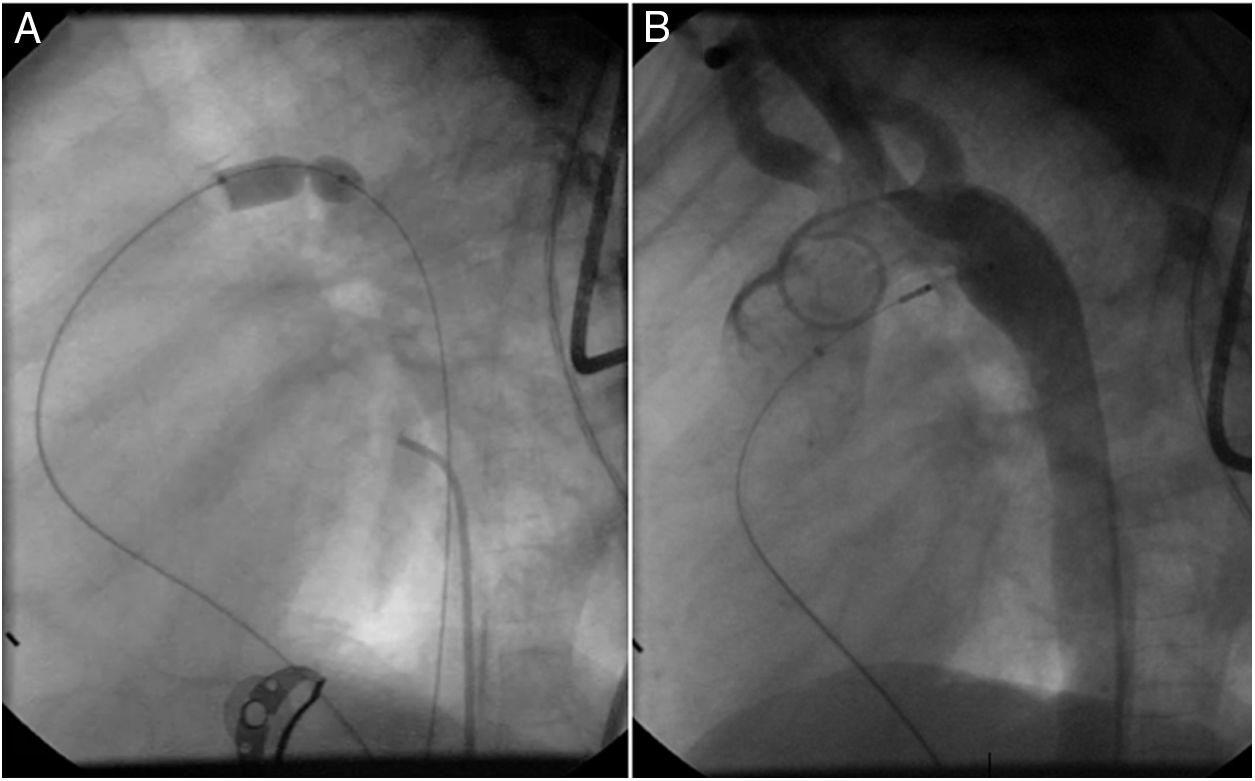
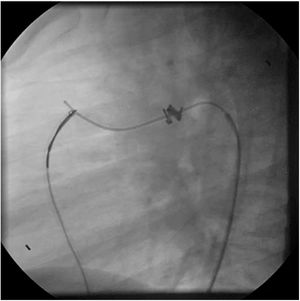
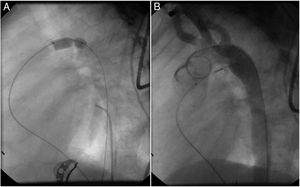
Both patients with history of previous PDA closure were successfully treated. The first was a seven year-old child who had a coil implanted at age three. The procedure was technically difficult, requiring a guidewire capture using a snare catheter to go through the residual PDA (Figure 11), and an Amplatzer duct occluder II 3x4 mm device was implanted successfully. The second patient was a 19 month-old child with PDA and pulmonary hypertension after an unsuccessful surgical ligation. In this case, sizing with a Tyshak balloon was required before implanting an Amplatzer duct occluder II 3x4 mm device (Figure 12).
Effective ductal closure was achieved in every treated patient, as confirmed by a post-implantation aortogram, with three patients (1.4%) presenting residual shunting. In these patients, the shunting was considered to be trivial, no second cardiac catheterization was ever attempted, and there was no described hemolysis. The residual shunting had no significant relationship to the size or shape of the ductus, while being more frequent in patients who were treated with coils.
ComplicationsNo major complications were reported during the procedure. No significant differences in complication rates between different devices were noted. In one patient with a small patent ductus arteriosus, a 6x5 mm coil was initially placed but migrated to the main pulmonary artery after being set. The aortogram revealed a larger than previously measured ductus arteriosus, due to likely vessel spasm. The ductus was then closed using an 8x6 mm Amplatzer duct occluder I device, with a good end result.
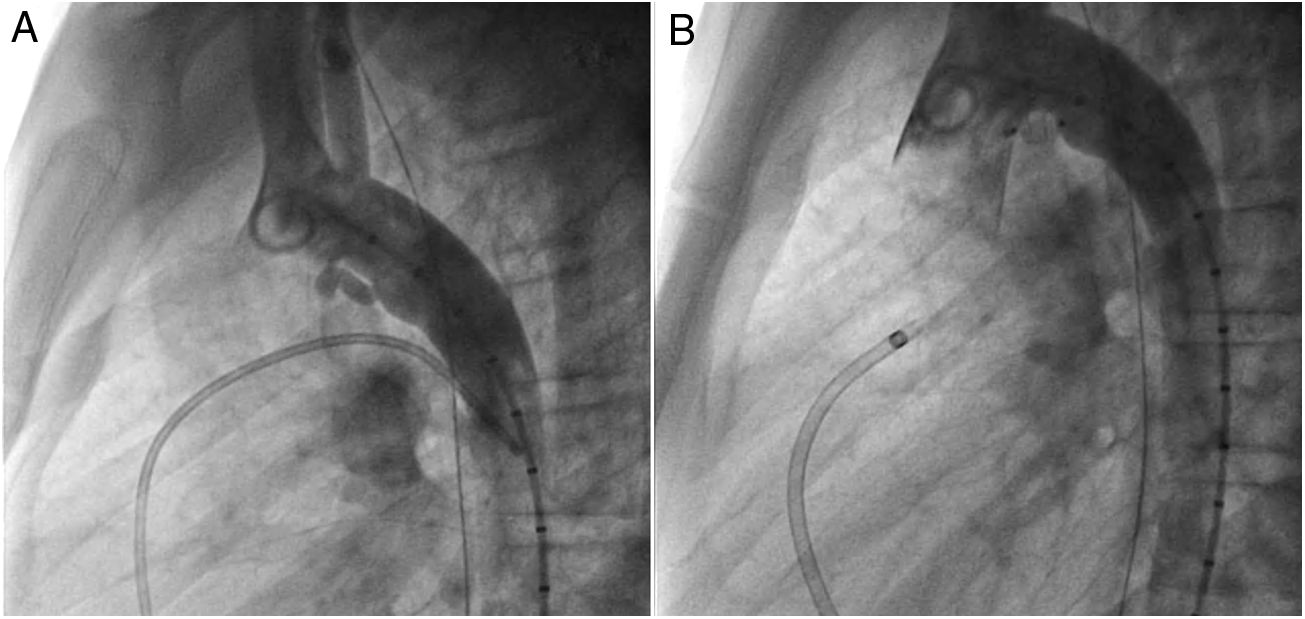
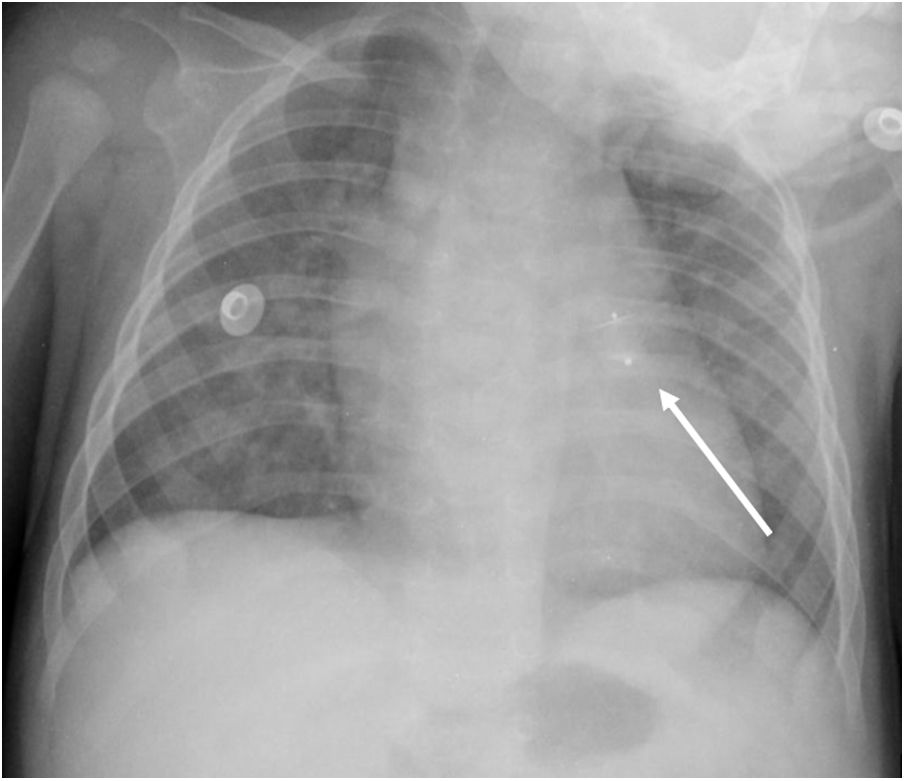
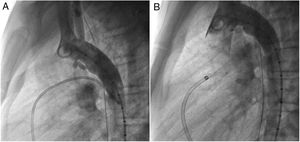
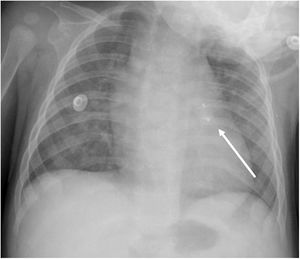
An early post-procedure complication rate of 1.8% was found (four patients). Three patients presented isolated loss of arterial pulse and were successfully treated with fibrinolytics. The most severe complication was in a seven month-old patient who presented device embolization 48 hours after the procedure. In this infant with a history of prematurity (born at 26 weeks of gestation), an Amplatzer duct occluder II Additional Sizes (5x6 mm) had been implanted. Loss of lower limb arterial pulse was noted a few hours after the procedure. A vascular doppler ultrasound confirmed occlusion of the femoral artery and thrombolysis with recombinant tissue plasminogen activator was started. After 48 hours, a chest radiograph revealed the device had migrated (Figure 13), with computed tomography confirming the device was at an inferior lobar branch of the left pulmonary artery. This required urgent surgical removal of the Amplatzer duct occluder II Additional Sizes and PDA ligation, which were both uneventful.
DiscussionTo the authors knowledge, this is the largest Portuguese series concerning PDA Nit-Occlud devices, while also being one of the largest globally. For example, a recently published multicenter prospective study in the USA enrolled a total of 184 patients with a 12-month follow-up.14 Our study, with the limitations arising from being a retrospective analysis, presents data from 139 patients who were treated with a Nit-Occlud coil in a single center. Our global average follow-up was 49 months (minimum one month, maximum 12 years). Another technical aspect the authors wish to highlight is that in about 20% of our patients an arterio-venous circuit was established prior to device implantation.
After the first percutaneous PDA closure by Porstmann in 1967,1 several devices and techniques have been developed for clinical practice.9 Currently approved coils include the Nit-Occlud or the Flipper® coil (Cook® Medical, IN, USA). Duct occluders suitable for PDA closure include the Amplatzer Duct Occluder, the Occlutech® Duct Occluder (Occlutech, Helsingborg, Sweden), the CeraFlex™ PDA Occluder (LifeTech Scientific, Shenzhen, China) or the Cardi-O-Fix Occluder (Starway Medical Technology Inc., Beijing, China). The Nit-Occlud is a nitinol coil with two cones of spirals designed with stiffer aortic windings to prevent “pull-through” into the pulmonary artery. As it was easily available at our center, and considering its excellent efficacy and safety, it was the chosen device for closure of small- to medium-sized PDA.14 Concerning moderate- to large-sized ductus, the ADO was considered a good therapeutic option at our center. Also made from a Nitinol wire mesh, and supported by a 20-year track record, it presents as a safe, effective and well-studied device.15
Currently, the transcatheter approach is a globally safe and attractive alternative to surgical ligation in most patients, allowing for shorter hospital stays and avoiding the thoracotomy scar, with minimal discomfort or pain for the child.16,17 With increasing experience, complications have been reduced to a minimum, while not being completely eliminated.4,12,18 Acute arterial injury is usually the most common complication in pediatric series,19 with device embolization, narrowing of the pulmonary artery, aortic obstruction, hemolysis, or infective endocarditis accounting only for a minority of cases.20–23 We reported four cases of loss of arterial pulse, with one of them developing a more severe complication 48 hours after the procedure.
In our series, a very high rate of procedural success was described, regardless of patient age, ductal size, or device used. The 1.4% rate of residual shunting in our patients is similar to other larger series.12,24,25 The major factors for success of the transcatheter approach include vascular accessibility, morphology of the ductus, imaging modality, and, perhaps most importantly, adequate selection of the device.26
Percutaneous treatment in extremely premature infants is technically difficult, and therefore, often not considered as an alternative to surgery. Recent reports have presented promising data, with these patients showing early recovery and less of a need for short-term respiratory support compared to surgical ligation (still the standard of care in most centers).5 We hypothesize that with growing experience and the advent of new devices specifically for this population, percutaneous PDA closure will become the mainstay of treatment for all patients regardless of age or weight.
LimitationsThis was a retrospective single center study with a medium overall sample size. While all major complications were reported, minor procedural incidents or technical difficulties might have been missed in electronic medical records. All patients were referred by their attending pediatric cardiologist and, since our center is tertiary, there is possible referral bias. Patient scheduling and selection of devices were at the discretion of the operators. All follow-up echocardiograms were performed by the attending physician.
ConclusionOverall, percutaneous PDA closure was safe and effective in this setting, with a low global complication rate and similar outcomes to most equivalent centers.
Financial supportThis research received no specific grant from any funding agency, commercial or not-for-profit sectors
Conflicts of interestThe authors have no conflicts of interest to declare.
None.