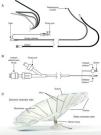
The Parachute is a novel left ventricular (LV) partitioning device that is deployed percutaneously in the left ventricle in patients with anteroapical regional wall motion abnormalities, dilated LV and systolic dysfunction after anterior myocardial infarction (MI). The implantable device is a partitioning membrane that isolates the dysfunctional region of the ventricle and decreases chamber volume.
Data from the first-in-human clinical trial – the Percutaneous Ventricular Restoration in Chronic Heart Failure (PARACHUTE) trial– has shown that this new device is associated with significant and sustained LV volume reduction and improvement in LV hemodynamics and functional capacity in the 12 months after implantation, with a relatively low rate of clinical events, indicating that it may have a beneficial effect in the treatment of ischemic heart failure.
We aim to describe our initial experience with implantation of the Parachute LV partitioning device and its short-term safety, defined as the successful delivery and deployment of the device.
O dispositivo Parachute é um dispositivo inovador, implantado percutaneamente no ápex do ventrículo esquerdo (VE) em doentes com discinésia ou acinésia antero-apical, dilatação e disfunção sistólica do VE, no decurso de um enfarte do miocárdio envolvendo a artéria descendente anterior esquerda. O dispositivo corresponde a uma membrana de partição, desenhada de forma a isolar a região acinética ou discinética e reduzir o volume do VE.
O estudo piloto - the Percutaneous Ventricular Restoration in Chronic Heart Failure (PARACHUTE) trial - mostrou que o dispositivo está associado a uma redução significativa e sustentada do volume do VE, com melhoria hemodinâmica e da capacidade funcional. Estes resultados positivos mantiveram-se durante os 12 meses após a implantação, com uma taxa relativamente baixa de eventos adversos relacionados com o dispositivo. Estes dados indicam que o dispositivo Parachute poderá ser benéfico no tratamento de doentes com insuficiência cardíaca com dilatação e disfunção sistólica do VE secundária a cardiopatia isquémica.
Os autores pretendem demonstrar a experiência inicial com a implantação do dispositivo, assim como a segurança do mesmo a curto-prazo relativamente à entrega e deposição do dispositivo no ápex do VE.
creatinine kinase
creatinine kinase MB isoenzyme
cardiac resynchronization therapy
computed tomography
expanded polytetrafluoroethylene
French
heart failure
international normalized ratio
left anterior descending artery
left ventricle/left ventricular
left ventricular ejection fraction
left ventricular systolic dysfunction
major adverse cardiac events
myocardial infarction
New York Heart Association
Heart failure (HF) is one of the major unsolved problems in cardiology and one of the leading causes of death. In the majority of cases, the clinical syndrome of HF results from left ventricular systolic dysfunction (LVSD). Obstructive coronary artery disease is the major cause of LVSD, responsible for almost 70% of cases.1
Post-myocardial infarction (MI) HF is more common in patients with an anterior infarction. The anteroapical region is a particularly vulnerable region of the LV for dilatation because of its thinner structure and greater curvature. When this region undergoes expansion and thinning, even small segmental elongation can greatly increase the radius of curvature, resulting in a large increase in wall stress. The associated LV remodeling leads to increased myocardial oxygen consumption secondary to increased wall stress, increased neurohormone and cytokine levels, afterload mismatch, and subendocardial hypoperfusion. This results in an inefficient, dilated and failing LV, leading to HF symptoms. LV remodeling is a progressive and self-perpetuating process, and has long been recognized as a strong predictor of mortality and morbidity after MI.2
There are a number of treatment options available to minimize symptoms and to slow disease progression. A combination of lifestyle changes and drug therapy – the foundation of almost all HF treatment regimens – can improve both survival rates and quality of life. However, these regimens have certain limitations, especially when LV anteroapical wall motion abnormality is present. Currently, the medical community recognizes that pharmacologic therapy has been optimized virtually as far as possible, and morbidity and mortality in post-MI HF patients continue to be excessive despite advances in medical treatment. Patients with advanced heart failure may become candidates for other interventions, including surgery and implantable devices.
Several surgical approaches have been advocated for LV volume reduction, including surgical ventricular remodeling (Dor procedure) and partial left ventriculectomy (Batista procedure). Data from surgical trials indicate that the described procedures significantly reduce LV volumes,3,4 but with mixed effects on functional capacity and quality of life. Favorable outcomes have only been reported in selected patients.5
Against this background, a novel percutaneous implantable device has been developed – the Parachute LV partitioning device (CardioKinetix, Inc, Menlo Park, CA) – which is deployed in the LV of patients with anteroapical regional wall motion abnormalities (akinesia or dyskinesia) following an anterior MI. The purpose of the Parachute device is to partition the LV and isolate the dysfunctional anteroapical region, reduce both systolic and diastolic volumes, decrease myocardial wall stress, and thereby improve LV hemodynamics.
Pre-clinical studies in an animal model of MI were performed before implantation of the Parachute device in humans, and indicated that the device has beneficial acute and medium-term effects on LV function, as measured by a decrease in LV volumes and end-diastolic pressure, increased cardiac output, and improvement in left ventricular ejection fraction (LVEF).6
Data from the first-in-human clinical trial (a single-arm, prospective, nonrandomized, multicenter trial – the Percutaneous Ventricular Restoration in Chronic Heart Failure (PARACHUTE) trial – showed that the Parachute implant was safe and feasible and improved LV hemodynamics, functional class and exercise capacity in the 12 months following the procedure.7 Recently, data from the 24-month follow-up have been released. The benefits achieved at 12 months were maintained at 24 months, associated with a relatively low rate of adverse events.
A dual-arm (Parachute vs. optimal medical therapy), open-label, multicenter registry designed to evaluate the Parachute implant in ischemic heart failure patients in 14 centers across Europe is ongoing – the Percutaneous Ventricular Restoration in Chronic Heart Failure (PARACHUTE, Cohort B) trial. The primary endpoint of the trial is the assessment of safety, defined as the successful delivery and deployment of the Parachute implant through 6-month follow-up without the occurrence of major adverse cardiac events (MACE) related to the investigational device. Other key endpoints include changes in LV volume indices (LV end-systolic and end-diastolic volumes, measured by transthoracic echocardiography) and in exercise tolerance (assessed by the six-minute walk test).
We aim to describe the initial experience of our center with the Parachute implant and its short-term safety, defined as the successful delivery and deployment of the device.
MethodsPatientsBetween November 2011 and May 2012, a total of five patients were selected for implantation. Major inclusion criteria were: (1) anteroapical wall motion abnormality (akinesia or dyskinesia) of the LV following an anterior MI, detected by transthoracic echocardiography; (2) LVEF ≥15% and ≤40% and evidence of LV dilatation; (3) diagnosis of HF with a minimum of six months prior to device implantation; (4) New York Heart Association (NYHA) class ≥II/VI despite optimal medical therapy (according to the current guidelines for the treatment of chronic heart failure8,9); (5) eligibility for cardiac surgery; (6) age ≥18 years and ≤79 years; (7) suitable LV anatomy to accommodate the device, assessed by computed tomography (CT); and (8) signed written informed consent. Major exclusion criteria were: (1) MI, revascularization procedure, permanent pacemaker, cardiac resynchronization therapy (CRT) or implantable cardioverter-defibrillator implantation within 60 days prior to enrolment; (2) stroke or transient ischemic accident within 6 months prior to enrolment; (3) thrombus in the LV or left atrium; (4) history of bleeding or known blood disorder; (5) risk of contrast-induced nephropathy; (6) moderate to severe valve disease (stenosis or regurgitation); and (7) previous aortic valve replacement or repair.
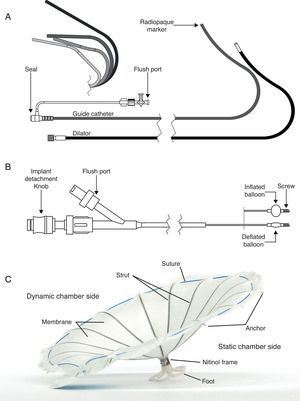
Device componentsThe Parachute device consists of three components: the access system, the delivery system, and the implant. The device components are represented in Figure 1.
The access system consists of a guide catheter and dilator to provide access to the LV. A broad range of catheter shapes are available so that an array of remodeled ventricle shapes can be treated. The guide catheter is available in 14 French (Fr) and 16 Fr, and the internal lumen is sized to accommodate a collapsed Parachute implant.
The delivery system is used to deliver and position the Parachute implant in the LV. The central lumen provides a channel for the torque shaft, at the distal end of which is a screw that engages the Parachute implant. By rotating the detachment knob at the proximal end of the delivery catheter, the Parachute implant can be attached or detached. The balloon is designed to push against the struts of the implant when inflated, to ensure full expansion and engagement of struts into the tissue of the LV wall.
The Parachute implant consists of a self-expanding nitinol frame, an expanded polytetrafluoroethylene (ePTFE) occlusive membrane, and an atraumatic (pebax polymer) foot. The umbrella-shaped nitinol frame has 16 struts and the tip of each strut ends in a 2mm anchor. The purpose of the anchors is to engage the tissue, stabilize the implant, and prevent dislodgment and migration after it is detached from the delivery catheter. Once the implant is expanded, the membrane provides a barrier to seal off the static chamber on the distal side of the implant.
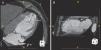
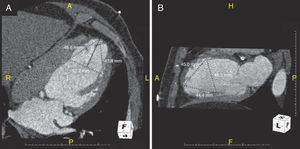
Eight different Parachute sizes are available for implantation – 65mm, 75mm, 85mm and 95mm (regular and short foot). Implant sizing is previously assessed by CT imaging. The attachment zone (diameter and height) is measured at end-diastole, 40mm from the apex in both short- and long-axis views or in two perpendicular long-axis views, with particular attention to its position relative to the papillary muscles (Figure 2). It is recommended that the Parachute diameter should exceed the largest LV diameter by 30–60%. Cardiac CT scanning allows accurate identification of the LV apex anatomy and LV morphology. It also helps to identify any LV wall calcification within the implant zone. The presence of calcification at the device attachment level of the LV precludes the implantation as the device could be at risk of migration due to the inability of the anchors to adequately engage in the muscle.
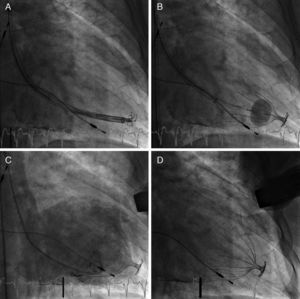
Parachute device implantation techniquePatients were prepared as for left heart catheterization according to the hospital's standard procedures. The procedure was performed using local anesthesia and mild conscious sedation. Left ventriculography was performed in two projections (left and right anterior oblique) to confirm LV sizes measured by baseline echocardiogram and CT. In all patients, implantation was performed via the right femoral artery. The collapsed Parachute implant was attached to the delivery catheter, and advanced retrogradely through the guide catheter across the aortic valve and positioned in the LV apex (Figure 3A). An LV angiogram was performed to assess appropriate positioning. Once in place, the Parachute implant was expanded by the compliant balloon located proximal to the screw connector (Figure 3B), and released using the distal screw mechanism. Control left ventriculography was performed to assess the device's position and any residual leak between the walls of the LV and the device (Figure 3C).
Implantation of the Parachute device showing (A) positioning of the collapsed implant at the left ventricular apex; (B) expansion of the Parachute implant by inflation of the compliant balloon; (C) final left ventriculography showing the correctly positioned Parachute implant partitioning the left ventricle into a static and a dynamic chamber; and (D) final angiogram without contrast showing the Parachute implant positioned at the left ventricular apex.
During the procedure, all patients received intravenous boluses of unfractionated heparin in sufficient dosage to prolong activated clotting time to >250s. At the time of implantation and on discharge, all patients received optimal therapy for chronic heart failure consisting of an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, beta-blocker, and diuretic. In addition, all patients received aspirin (100–150mg/day) for a minimum of 12 months post-procedure, and were placed on anticoagulation therapy (warfarin or acenocoumarol) for a minimum of six months post-implantation. An international normalized ratio (INR) ≥2 must have been achieved prior to discontinuing unfractionated heparin (or low molecular weight heparin) after the procedure, and INR between 2.0 and 3.0 must be maintained after hospital discharge.
EchocardiographyComplete two-dimensional transthoracic echocardiographic and Doppler examinations were performed in all patients, both at baseline and at discharge from the hospital. LV ejection fraction and LV volumes were determined from the apical 4- and 2-chamber views using Simpson's biplane formula. The competence of the Parachute implant seal was evaluated by color Doppler echocardiography performed at the site of the implant attachments to the myocardium. The extent of leakage was assessed semiquantitatively, and graded by the core echo lab as no leakage, or mild, moderate, or severe leakage.
Symptoms and functional capacityHeart failure symptoms were evaluated at baseline using the NYHA classification, and functional capacity was assessed using the standard six-minute walk test, at the same time.
Laboratory assessmentSerum creatinine and blood urea nitrogen were measured at baseline, 24 hours after implantation, and at discharge, to assess the impact of the procedure on renal function. Additionally, troponin T, creatinine kinase (CK), and creatinine kinase MB isoenzyme (CK-MB) were measured within 12 hours post-procedure to assess possible myocardial damage associated with the procedure. If CK was twice the upper limit of normal and CK-MB was above normal, the analysis was repeated every eight hours until nadir was documented.
ResultsBaseline demographic, echocardiographic, functional capacity and laboratory data of the patients included in the study are shown in Tables 1–4.
Baseline demographic data.
| Gender (male), n | 5 |
| Age (years), mean±SD | 61±8 |
| Cardiovascular risk factors | |
| Diabetes, n | 2 |
| Hypertension, n | 2 |
| Dyslipidemia, n | 5 |
| Previous cardiovascular events | |
| Previous LAD MI, n | 5 |
| Previous PCI or CABG, n | 5 |
| Previous stroke or TIA, n | 2 |
| Atrial fibrillation, n | 1 |
| Previous PM implantation, n | 1 |
| Comorbidities | |
| Tracheal stenosis, n | 1 |
| OSA, n | 1 |
| Pulmonary hypertension, n | 2 |
CABG: coronary artery bypass grafting; LAD: left anterior descending artery; MI: myocardial infarction; OSA: obstructive sleep apnea; PCI: percutaneous coronary intervention; PM: pacemaker; TIA: transient ischemic attack.
Baseline echocardiographic data.
| LVEF, % (mean±SD) | 29±4 |
| LVEDD, mm (mean±SD) | 69±9 |
| LVEDV, ml (mean±SD) | 256±75 |
| PASP, mmHg (mean±SD) | 40±12 |
LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; PASP: pulmonary artery systolic pressure.
A 16-Fr catheter was used in four patients; in the other patient a 14-Fr catheter was used due to peripheral arterial disease. A 95mm implant was used in three patients, an 85mm device in one, and a 75mm device in the other patient. An optimal apical position of the implant was achieved in all patients but one. In this patient, the Parachute device was positioned in a more posterolateral location due to difficulty in accessing the LV apex. No catheter or device malfunctions were noted. Procedure time averaged 62±18min, with a mean fluoroscopy time of 15±4min. A mean of 178±41ml of contrast agent was used per patient. No complications ensued during the procedure. In all patients, vascular access site closure was performed using percutaneous suture.
Regarding in-hospital outcome, only one event was noted, consisting of minor bleeding of the vascular access site. Serum creatinine and blood urea nitrogen showed no significant changes at 24 hours following the procedure or at discharge. CK, CK-MB, and troponin T were consistently within reference ranges, indicating that no myocardial damage had occurred during implantation.
Data on echocardiographic characteristics after Parachute implantation are shown in Table 5. There was a significant reduction in end-diastolic LV volumes, and LV ejection fraction improved slightly after Parachute implantation compared to baseline values. On discharge, there was no leakage between the static and dynamic LV chamber in four patients, while in one patient a mild leakage was noted.
DiscussionThe most direct approach to revert the remodeling itself is a mechanical intervention to decrease LV volume or to constrain the ventricle from further enlargement. Several surgical approaches have been advocated in patients with a dilated LV, including partial left ventriculectomy (Batista procedure) and surgical ventricular remodeling (Dor procedure). Of these procedures, only surgical ventricular remodeling has acceptable short- and long-term effects on mortality and morbidity. Even so, perioperative mortality is more than 5%, and overall 5-year survival is less than 70%.5 By contrast, LV volume reduction with the Parachute device provides a clinical advantage over the currently used mechanical interventions as it is implanted percutaneously, thus avoiding the early mortality and morbidity associated with surgical intervention.
In our patients, Parachute implantation was safe and feasible, with acceptable procedure and fluoroscopy times. There was a significant reduction in end-diastolic volume after implantation. Our patients had very large left ventricles, and hence there was considerable LV volume reduction after implantation. The fact that no device migration occurred during implantation suggests that the Parachute was implanted in a region where anchor penetration in the myocardium was sufficient to provide device stability.
Although current data indicates that the Parachute implant is safe and feasible and improves LV hemodynamics, functional class and exercise capacity in the 24 months following the procedure, longer follow-up is needed to put the Parachute into an appropriate clinical perspective.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.