Selecting patients for heart transplantation is challenging. We aimed to identify the most important risk predictors in heart failure and an approach to optimize the selection of candidates for heart transplantation.
MethodsAmbulatory patients followed in our center with symptomatic heart failure and left ventricular ejection fraction ≤40% prospectively underwent a comprehensive baseline assessment including clinical, laboratory, electrocardiographic, echocardiographic, and cardiopulmonary exercise testing parameters. All patients were followed for 60 months. The combined endpoint was cardiac death, urgent heart transplantation or need for mechanical circulatory support, up to 36 months.
ResultsIn the 263 enrolled patients (75% male, age 54±12 years), 54 events occurred. The independent predictors of adverse outcome were ventilatory efficiency (VE/VCO2) slope (HR 1.14, 95% CI 1.11-1.18), creatinine level (HR 2.23, 95% CI 1.14-4.36), and left ventricular ejection fraction (HR 0.96, 95% CI 0.93-0.99). VE/VCO2 slope was the most accurate risk predictor at any follow-up time analyzed (up to 60 months). The threshold of 39.0 yielded high specificity (97%), discriminated a worse or better prognosis than that reported for post-heart transplantation, and outperformed peak oxygen consumption thresholds of 10.0 or 12.0 ml/kg/min. For low-risk patients (VE/VCO2 slope <39.0), sodium and creatinine levels and variations in end-tidal carbon dioxide partial pressure on exercise identified those with excellent prognosis.
ConclusionsVE/VCO2 slope was the most accurate parameter for risk stratification in patients with heart failure and reduced ejection fraction. Those with VE/VCO2 slope ≥39.0 may benefit from heart transplantation.
A seleção de doentes para transplantação cardíaca é difícil. Procurámos identificar os preditores de risco mais relevantes na insuficiência cardíaca e uma abordagem para aprimorar a seleção de candidatos a transplantação.
MétodosDoentes sintomáticos com insuficiência cardíaca e fração de ejeção ventricular esquerda ≤ 40%, ambulatórios, seguidos no nosso centro, completaram prospetivamente uma avaliação basal abrangente, inclusive parâmetros clínicos, laboratoriais, eletrocardiográficos, ecocardiográficos e prova de esforço cardiorrespiratória; foram seguidos por 60 meses. Endpoint combinado: morte de causa cardíaca, transplantação urgente ou necessidade de assistência mecânica, até aos 36 meses.
ResultadosNos 263 doentes incluídos (75% homens, 54 ±12 anos) ocorreram 54 eventos. O declive da eficiência ventilatória (declive VE/VCO2) (HR 1,14, IC 95% 1,11-1,18), a creatinina (HR 2,23, IC 95% 1,14-4,36) e a fração de ejeção ventricular esquerda (HR 0,96, IC 95% 0,93-0,99) foram preditores independentes de eventos. O declive VE/VCO2 foi o melhor preditor em qualquer período analisado (até aos 60 meses). O limiar 39,0 apresentou elevada especificidade (97%), discriminou um prognóstico melhor ou pior do que o reportado no pós-transplante cardíaco e superou os limiares 10,0 ou 12,0 mL/kg/min de consumo de oxigénio de pico. Em doentes de baixo risco (declive VE/VCO2 <39,0) o sódio, a creatinina e a variação no exercício da pressão parcial de dióxido de carbono expirado identificaram aqueles com excelente pronóstico.
ConclusõesO declive VE/VCO2 foi o melhor preditor de risco em doentes com insuficiência cardíaca e fração de ejeção reduzida. Doentes com declive VE/VCO2 ≥39,0 poderão beneficiar de transplantação cardíaca.
variation of end-tidal carbon dioxide partial pressure
confidence interval
cardiopulmonary exercise testing
heart failure
heart transplantation
integrated discrimination improvement
International Society for Heart and Lung Transplantation
left ventricular ejection fraction
net reclassification improvement
peak oxygen consumption
ventilatory efficiency slope
A wide variety of predictors of adverse outcome in heart failure (HF) have been described and it can be difficult to choose the most appropriate tools in clinical practice.1,2 Risk stratification should be as accurate as possible, particularly when selecting patients for heart transplantation (HTX), as procedure-related morbidity and mortality are non-negligible and it cannot be offered to all candidates due to the shortage of donors.3 For ambulatory patients, both the American Heart Association and the International Society for Heart and Lung Transplantation (ISHLT) recommend the use of peak oxygen consumption (VO2 max) achieved in cardiopulmonary exercise testing (CPET), with optional use of risk scores in gray zones; VO2 max <10-12 ml/kg/min is considered a listing criterion for HTX.4,5 We believe that risk stratification and referral criteria for HTX can be improved using simple parameters. Additional CPET variables have shown to be accurate for risk stratification, particularly the ventilatory efficiency (VE/VCO2) slope.6–8 However, most studies that highlight the value of ergospirometric parameters have focused mainly on clinical and CPET data, without comprehensive assessment of other parameters, and few had long-term follow-up.6–9 Identifying robust criteria for selecting patients for HTX should be based on a comprehensive prospective clinical and complementary assessment.
Our aims were to identify the most accurate predictors of adverse events in non-transplanted patients with HF and an approach to optimize the selection of patients for HTX.
MethodsThe investigation conforms to the principles outlined in the Declaration of Helsinki. The institutional ethics committee approved the study protocol.
Patient selection and complementary assessmentThis single-center analysis included all patients with HF with left ventricular ejection fraction (LVEF) ≤40%, in New York Heart Association class II or III, followed in the heart failure clinic of our institution between 2000 and 2009. All patients referred to the heart failure clinic underwent a comprehensive complementary assessment. Clinical, laboratory, electrocardiographic, echocardiographic, and CPET data were prospectively collected; all these exams were performed within a period of one month in each patient.
Patients aged <18 years and those with planned percutaneous coronary revascularization or cardiac surgery, exercise-limiting comorbidities (cerebrovascular disease, musculoskeletal impairment, or severe peripheral vascular disease), previous HTX, or failure to achieve the anaerobic threshold were excluded.
Cardiopulmonary exercise testingMaximal symptom-limited treadmill CPET was performed using the modified Bruce protocol (GE Marquette Series 2000 treadmill). Gas analysis was preceded by calibration of the equipment. Minute ventilation, oxygen uptake and carbon dioxide production were acquired breath-by-breath, using a SensorMedics Vmax 229 gas analyzer. Patients were encouraged to exercise until the respiratory exchange ratio (ratio between carbon dioxide production and oxygen consumption) was ≥1.10. VO2 max was defined as the highest 30-s average achieved during exercise and was normalized for body mass, corrected for fat-free mass in obese patients (body mass index >30 kg/m2). The ventilatory threshold was determined by combining the standard methods (V-slope and ventilatory equivalents).10 The VE/VCO2 slope was calculated by least squares linear regression, using data acquired throughout the exercise session.10 Several composite CPET parameters were also calculated.
Follow-up and endpointAll patients were followed for 60 months from the date of completion of the above-mentioned complementary exams. Patients were assessed for the occurrence of death, HTX or the need for mechanical circulatory support. Data were obtained from outpatient clinic visits and review of medical charts, and were complemented by a standardized telephone interview with all patients at 12, 36 and 60 months of follow-up.
The combined endpoint of cardiac death, urgent HTX (occurring during an unplanned hospitalization for worsening of HF, requiring inotropes) or the need for mechanical circulatory support was analyzed.
Statistical analysisCategorical data are presented as frequencies (percentages) and continuous variables as mean (standard deviation) or median (25th-75th percentile), as appropriate. Continuous variables were analyzed using the Student's t test, or the Mann-Whitney test when normality was not verified by the Kolmogorov-Smirnov test; categorical variables were analyzed using the chi-squared or Fisher's exact tests.
Univariate and multivariate Cox regression models were applied to time until the combined endpoint, considering the follow-up times of 12, 36 and 60 months. A complete list of the tested variables and a detailed description of the univariate and multivariate analysis are presented as supplementary material (Supplementary Tables 1 and 2).
The 36-month follow-up period was analyzed to identify a possible approach for selecting patients for HTX.7,8,11 To identify the most accurate individual predictor from the multivariate model, the variable with the highest area under the curve (AUC) on receiver operating characteristic (ROC) analysis (VE/VCO2 slope, post hoc) was selected. To assess the additive value of other predictors to VE/VCO2 slope, the AUC of VE/VCO2 slope was compared with the AUC of the model including all predictors of adverse outcome. The DeLong test was used to compare two correlated ROC curves. In addition, continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) measures for censored data were calculated.12
The best cut-off value of VE/VCO2 slope for identifying high-risk patients was calculated using the martingale residuals. Since HTX can only be offered to a small subgroup of HF patients, we identified another cut-off value that minimized the rate of misclassified high-risk patients, even if sensitivity decreased to reasonable levels.3 We thus identified a VE/VCO2 slope threshold that provided a high specificity (at least 90%) with at least 50% sensitivity, using the inverse probability of censoring weighting approach.13 Subgroups of high- and low-risk patients were created accordingly. Event-free survival rates of both subgroups were estimated using the Kaplan-Meier method and compared using the log-rank test. To assess whether the identified VE/VCO2 slope threshold is suitable as a potential indication for HTX, two analyses were carried out. Firstly, survival rates of high- and low-risk subgroups of our cohort were compared with survival rates after HTX reported by the ISHLT transplant registry (quarterly data report on survival rates for orthotopic HTX performed in Europe between October 1, 2011 and September 30, 2015), using a previously reported method.9,14 Since the ISHLT reports overall survival, we studied (for this analysis only) time to death from any cause, right censoring in the event of urgent or non-urgent HTX.15 If the 95% confidence intervals (CI) of estimated survival 36 months after HTX reported by the ISHLT did not overlap with those of high- and low-risk subgroups of our cohort, based on the VE/VCO2 slope threshold, this can be taken as evidence of significant difference.7,15 Secondly, the NRI and IDI were used to compare discretized VE/VCO2 slope and VO2 max, considering the combined endpoint for the analysis.
To further stratify prognosis in low-risk patients, high-risk patients were excluded from subsequent analysis and univariate and multivariate Cox regression models were fitted to the low-risk subgroup. Martingale residuals were used to identify cut-off values for the variables that remained in the model and subgroups of high- and low-risk patients were created accordingly. Event-free survival rates of the subgroups were estimated using the Kaplan-Meier method and compared using the Gehan-Breslow-Wilcoxon test.
The level of significance considered was α=0.05. Data were analyzed using SPSS for Windows, version 20.0 (IBM SPSS Inc., Chicago, IL) and the statistical program R Development Core Team (2014), R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria).
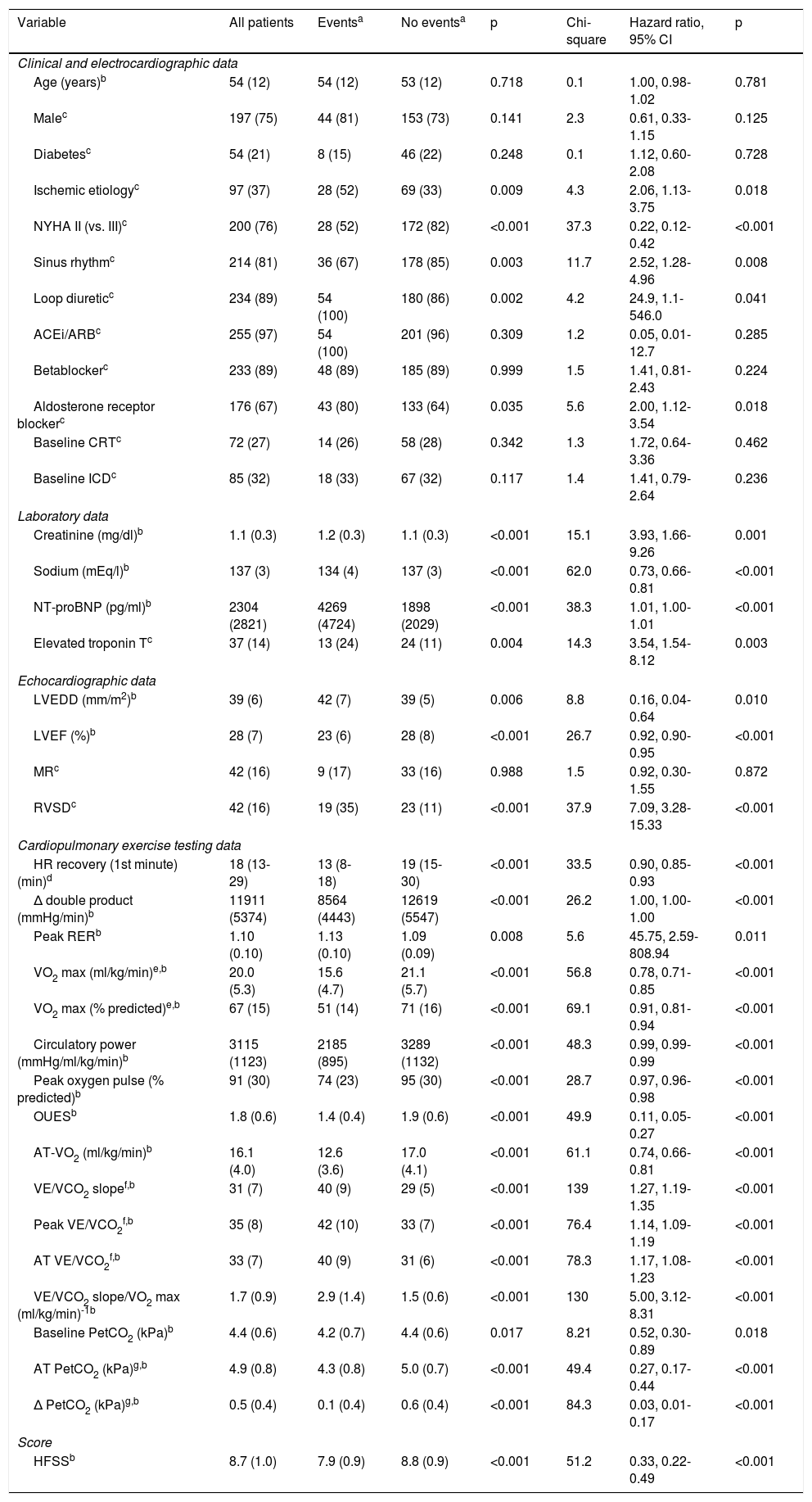
ResultsA total of 263 patients were included. The combined endpoint occurred in 54 patients (20.5%) within 36 months and in 69 (26.2%) within 60 months. The main baseline data are presented in Table 1, in which patients with and without events up to 36 months of follow-up are compared and the most important risk predictors identified in univariate Cox regression are presented. Complete data on baseline characteristics and univariate Cox regression are presented in Supplementary Tables 1 and 2, respectively.
Baseline data and univariate Cox regression analysis for predicting adverse events up to 36 months of follow-up.
| Variable | All patients | Eventsa | No eventsa | p | Chi-square | Hazard ratio, 95% CI | p |
|---|---|---|---|---|---|---|---|
| Clinical and electrocardiographic data | |||||||
| Age (years)b | 54 (12) | 54 (12) | 53 (12) | 0.718 | 0.1 | 1.00, 0.98-1.02 | 0.781 |
| Malec | 197 (75) | 44 (81) | 153 (73) | 0.141 | 2.3 | 0.61, 0.33-1.15 | 0.125 |
| Diabetesc | 54 (21) | 8 (15) | 46 (22) | 0.248 | 0.1 | 1.12, 0.60-2.08 | 0.728 |
| Ischemic etiologyc | 97 (37) | 28 (52) | 69 (33) | 0.009 | 4.3 | 2.06, 1.13-3.75 | 0.018 |
| NYHA II (vs. III)c | 200 (76) | 28 (52) | 172 (82) | <0.001 | 37.3 | 0.22, 0.12-0.42 | <0.001 |
| Sinus rhythmc | 214 (81) | 36 (67) | 178 (85) | 0.003 | 11.7 | 2.52, 1.28-4.96 | 0.008 |
| Loop diureticc | 234 (89) | 54 (100) | 180 (86) | 0.002 | 4.2 | 24.9, 1.1-546.0 | 0.041 |
| ACEi/ARBc | 255 (97) | 54 (100) | 201 (96) | 0.309 | 1.2 | 0.05, 0.01-12.7 | 0.285 |
| Betablockerc | 233 (89) | 48 (89) | 185 (89) | 0.999 | 1.5 | 1.41, 0.81-2.43 | 0.224 |
| Aldosterone receptor blockerc | 176 (67) | 43 (80) | 133 (64) | 0.035 | 5.6 | 2.00, 1.12-3.54 | 0.018 |
| Baseline CRTc | 72 (27) | 14 (26) | 58 (28) | 0.342 | 1.3 | 1.72, 0.64-3.36 | 0.462 |
| Baseline ICDc | 85 (32) | 18 (33) | 67 (32) | 0.117 | 1.4 | 1.41, 0.79-2.64 | 0.236 |
| Laboratory data | |||||||
| Creatinine (mg/dl)b | 1.1 (0.3) | 1.2 (0.3) | 1.1 (0.3) | <0.001 | 15.1 | 3.93, 1.66-9.26 | 0.001 |
| Sodium (mEq/l)b | 137 (3) | 134 (4) | 137 (3) | <0.001 | 62.0 | 0.73, 0.66-0.81 | <0.001 |
| NT-proBNP (pg/ml)b | 2304 (2821) | 4269 (4724) | 1898 (2029) | <0.001 | 38.3 | 1.01, 1.00-1.01 | <0.001 |
| Elevated troponin Tc | 37 (14) | 13 (24) | 24 (11) | 0.004 | 14.3 | 3.54, 1.54-8.12 | 0.003 |
| Echocardiographic data | |||||||
| LVEDD (mm/m2)b | 39 (6) | 42 (7) | 39 (5) | 0.006 | 8.8 | 0.16, 0.04-0.64 | 0.010 |
| LVEF (%)b | 28 (7) | 23 (6) | 28 (8) | <0.001 | 26.7 | 0.92, 0.90-0.95 | <0.001 |
| MRc | 42 (16) | 9 (17) | 33 (16) | 0.988 | 1.5 | 0.92, 0.30-1.55 | 0.872 |
| RVSDc | 42 (16) | 19 (35) | 23 (11) | <0.001 | 37.9 | 7.09, 3.28-15.33 | <0.001 |
| Cardiopulmonary exercise testing data | |||||||
| HR recovery (1st minute) (min)d | 18 (13-29) | 13 (8-18) | 19 (15-30) | <0.001 | 33.5 | 0.90, 0.85-0.93 | <0.001 |
| Δ double product (mmHg/min)b | 11911 (5374) | 8564 (4443) | 12619 (5547) | <0.001 | 26.2 | 1.00, 1.00-1.00 | <0.001 |
| Peak RERb | 1.10 (0.10) | 1.13 (0.10) | 1.09 (0.09) | 0.008 | 5.6 | 45.75, 2.59-808.94 | 0.011 |
| VO2 max (ml/kg/min)e,b | 20.0 (5.3) | 15.6 (4.7) | 21.1 (5.7) | <0.001 | 56.8 | 0.78, 0.71-0.85 | <0.001 |
| VO2 max (% predicted)e,b | 67 (15) | 51 (14) | 71 (16) | <0.001 | 69.1 | 0.91, 0.81-0.94 | <0.001 |
| Circulatory power (mmHg/ml/kg/min)b | 3115 (1123) | 2185 (895) | 3289 (1132) | <0.001 | 48.3 | 0.99, 0.99-0.99 | <0.001 |
| Peak oxygen pulse (% predicted)b | 91 (30) | 74 (23) | 95 (30) | <0.001 | 28.7 | 0.97, 0.96-0.98 | <0.001 |
| OUESb | 1.8 (0.6) | 1.4 (0.4) | 1.9 (0.6) | <0.001 | 49.9 | 0.11, 0.05-0.27 | <0.001 |
| AT-VO2 (ml/kg/min)b | 16.1 (4.0) | 12.6 (3.6) | 17.0 (4.1) | <0.001 | 61.1 | 0.74, 0.66-0.81 | <0.001 |
| VE/VCO2 slopef,b | 31 (7) | 40 (9) | 29 (5) | <0.001 | 139 | 1.27, 1.19-1.35 | <0.001 |
| Peak VE/VCO2f,b | 35 (8) | 42 (10) | 33 (7) | <0.001 | 76.4 | 1.14, 1.09-1.19 | <0.001 |
| AT VE/VCO2f,b | 33 (7) | 40 (9) | 31 (6) | <0.001 | 78.3 | 1.17, 1.08-1.23 | <0.001 |
| VE/VCO2 slope/VO2 max (ml/kg/min)-1b | 1.7 (0.9) | 2.9 (1.4) | 1.5 (0.6) | <0.001 | 130 | 5.00, 3.12-8.31 | <0.001 |
| Baseline PetCO2 (kPa)b | 4.4 (0.6) | 4.2 (0.7) | 4.4 (0.6) | 0.017 | 8.21 | 0.52, 0.30-0.89 | 0.018 |
| AT PetCO2 (kPa)g,b | 4.9 (0.8) | 4.3 (0.8) | 5.0 (0.7) | <0.001 | 49.4 | 0.27, 0.17-0.44 | <0.001 |
| Δ PetCO2 (kPa)g,b | 0.5 (0.4) | 0.1 (0.4) | 0.6 (0.4) | <0.001 | 84.3 | 0.03, 0.01-0.17 | <0.001 |
| Score | |||||||
| HFSSb | 8.7 (1.0) | 7.9 (0.9) | 8.8 (0.9) | <0.001 | 51.2 | 0.33, 0.22-0.49 | <0.001 |
Cardiac death, urgent heart transplantation or mechanical circulatory support up to 36 months of follow-up (n=54).
Values expressed as median (25th-75th percentile).
e,f,gOf these, only VO2 max, VE/VCO2 slope (entire exercise) and Δ PetCO2 were entered in the multivariate model, to avoid multicollinearity.
Δ double product: product of peak minus baseline heart rate and systolic blood pressure; Δ PetCO2: anaerobic threshold minus baseline end-tidal carbon dioxide partial pressure; ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; AT: anaerobic threshold; Chi-square: Wald chi-square value; CI: confidence interval; Circulatory power: product of peak oxygen consumption and systolic blood pressure; CRT: cardiac resynchronization therapy, with or without defibrillator; HFSS: Heart Failure Survival Score; HR: heart rate; HR recovery (1st minute): peak heart rate minus heart rate at first minute of recovery; ICD: implantable cardioverter-defibrillator; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; MR: moderate or severe mitral regurgitation; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association; OUES: oxygen uptake efficiency slope16; peak oxygen pulse: peak oxygen consumption/heart rate ratio; PetCO2: end-tidal carbon dioxide partial pressure; RER: respiratory exchange ratio (ratio between carbon dioxide production and oxygen consumption); RVSD: right ventricular systolic dysfunction; VO2 max: peak oxygen consumption; VO2 max (% predicted): based on Wasserman and Hansen's formula; VCO2: carbon dioxide production; VE: minute ventilation; VO2: oxygen consumption.
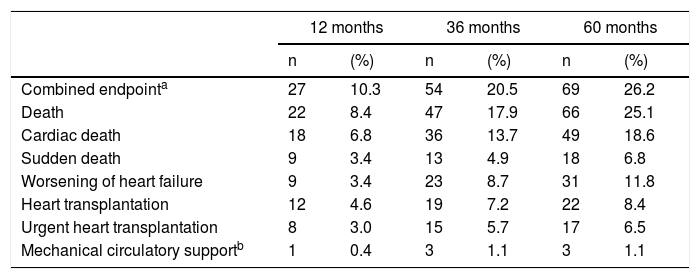
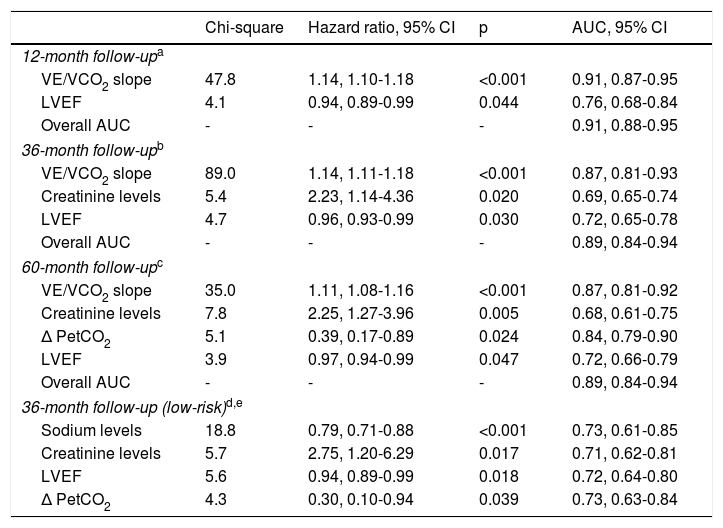
Cumulative adverse events occurring in different follow-up periods are presented in Table 2. The independent predictors of adverse events identified in multivariate Cox regression with follow-up times of 12, 36 and 60 months are presented in Table 3. At 36 months of follow-up, VE/VCO2 slope, creatinine levels and LVEF were independent predictors of adverse events. The VE/VCO2 slope had the highest Wald chi-square value in univariate and multivariate analysis at 36 months and was the parameter with the highest AUC in all multivariate models, considering 12, 36 and 60 months of follow-up. Specifically for the 36-month follow-up period, the AUC of the overall model including all predictors of adverse outcome did not differ significantly from the AUC of the model with VE/VCO2 slope alone (DeLong test p=0.103). In addition, the overall NRI was 63.4% (95% CI 33.5-93.4%) and the IDI was 0.019 (95% CI [-0.01]-[0.046]), when creatinine levels and LVEF were added to VE/VCO2 slope; as the 95% CI of IDI includes the null value, the improvement in model performance is negligible. Since the other predictors included in the model (creatinine levels and LVEF) did not add significantly to VE/VCO2 slope for risk prediction, only VE/VCO2 slope was selected to obtain a cut-off value for clinical use.
Adverse events at 12, 36 and 60 months of follow-up.
| 12 months | 36 months | 60 months | ||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Combined endpointa | 27 | 10.3 | 54 | 20.5 | 69 | 26.2 |
| Death | 22 | 8.4 | 47 | 17.9 | 66 | 25.1 |
| Cardiac death | 18 | 6.8 | 36 | 13.7 | 49 | 18.6 |
| Sudden death | 9 | 3.4 | 13 | 4.9 | 18 | 6.8 |
| Worsening of heart failure | 9 | 3.4 | 23 | 8.7 | 31 | 11.8 |
| Heart transplantation | 12 | 4.6 | 19 | 7.2 | 22 | 8.4 |
| Urgent heart transplantation | 8 | 3.0 | 15 | 5.7 | 17 | 6.5 |
| Mechanical circulatory supportb | 1 | 0.4 | 3 | 1.1 | 3 | 1.1 |
All patients were in Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles 1 or 2.17
Multivariate Cox regression.
| Chi-square | Hazard ratio, 95% CI | p | AUC, 95% CI | |
|---|---|---|---|---|
| 12-month follow-upa | ||||
| VE/VCO2 slope | 47.8 | 1.14, 1.10-1.18 | <0.001 | 0.91, 0.87-0.95 |
| LVEF | 4.1 | 0.94, 0.89-0.99 | 0.044 | 0.76, 0.68-0.84 |
| Overall AUC | - | - | - | 0.91, 0.88-0.95 |
| 36-month follow-upb | ||||
| VE/VCO2 slope | 89.0 | 1.14, 1.11-1.18 | <0.001 | 0.87, 0.81-0.93 |
| Creatinine levels | 5.4 | 2.23, 1.14-4.36 | 0.020 | 0.69, 0.65-0.74 |
| LVEF | 4.7 | 0.96, 0.93-0.99 | 0.030 | 0.72, 0.65-0.78 |
| Overall AUC | - | - | - | 0.89, 0.84-0.94 |
| 60-month follow-upc | ||||
| VE/VCO2 slope | 35.0 | 1.11, 1.08-1.16 | <0.001 | 0.87, 0.81-0.92 |
| Creatinine levels | 7.8 | 2.25, 1.27-3.96 | 0.005 | 0.68, 0.61-0.75 |
| Δ PetCO2 | 5.1 | 0.39, 0.17-0.89 | 0.024 | 0.84, 0.79-0.90 |
| LVEF | 3.9 | 0.97, 0.94-0.99 | 0.047 | 0.72, 0.66-0.79 |
| Overall AUC | - | - | - | 0.89, 0.84-0.94 |
| 36-month follow-up (low-risk)d,e | ||||
| Sodium levels | 18.8 | 0.79, 0.71-0.88 | <0.001 | 0.73, 0.61-0.85 |
| Creatinine levels | 5.7 | 2.75, 1.20-6.29 | 0.017 | 0.71, 0.62-0.81 |
| LVEF | 5.6 | 0.94, 0.89-0.99 | 0.018 | 0.72, 0.64-0.80 |
| Δ PetCO2 | 4.3 | 0.30, 0.10-0.94 | 0.039 | 0.73, 0.63-0.84 |
Excluding patients with VE/VCO2 slope ≥39.0.
AUC for individual variables and for the overall model are presented, for each follow-up time.
Δ PetCO2: anaerobic threshold minus baseline end-tidal carbon dioxide partial pressure; AUC: area under the receiving operating characteristic curve; Chi-square: Wald chi-square value; CI: confidence interval; LVEF: left ventricular ejection fraction; VCO2: carbon dioxide production; VE: minute ventilation.
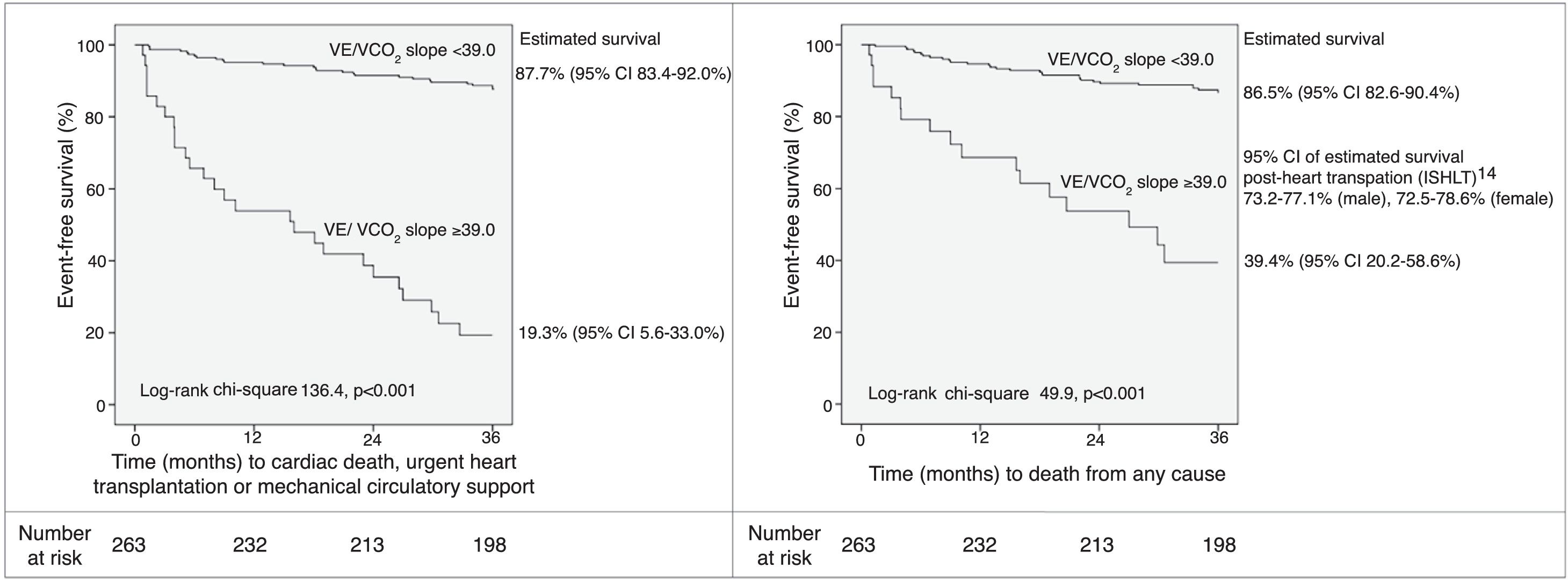
Based on the martingale residuals, the best estimated cut-off value for VE/VCO2 slope to identify patients at higher risk at 36 months of follow-up was 32.0 (specificity 80%, sensitivity 83%) (Supplementary Figure 1). However, a threshold of 39.0 provided higher specificity (97%), with 52% sensitivity. The estimated 36-month survival for high-risk (VE/VCO2 slope ≥39.0) and low-risk (VE/VCO2 slope <39.0) patients was significantly different (Figure 1).
Event-free survival up to 36 months of follow-up according to VE/VCO2 slope threshold of 39.0, and 95% CI of estimated survival for each subgroup. CI: 95% confidence interval; ISHLT: International Society for Heart and Lung Transplantation; VCO2: carbon dioxide production; VE: minute ventilation.
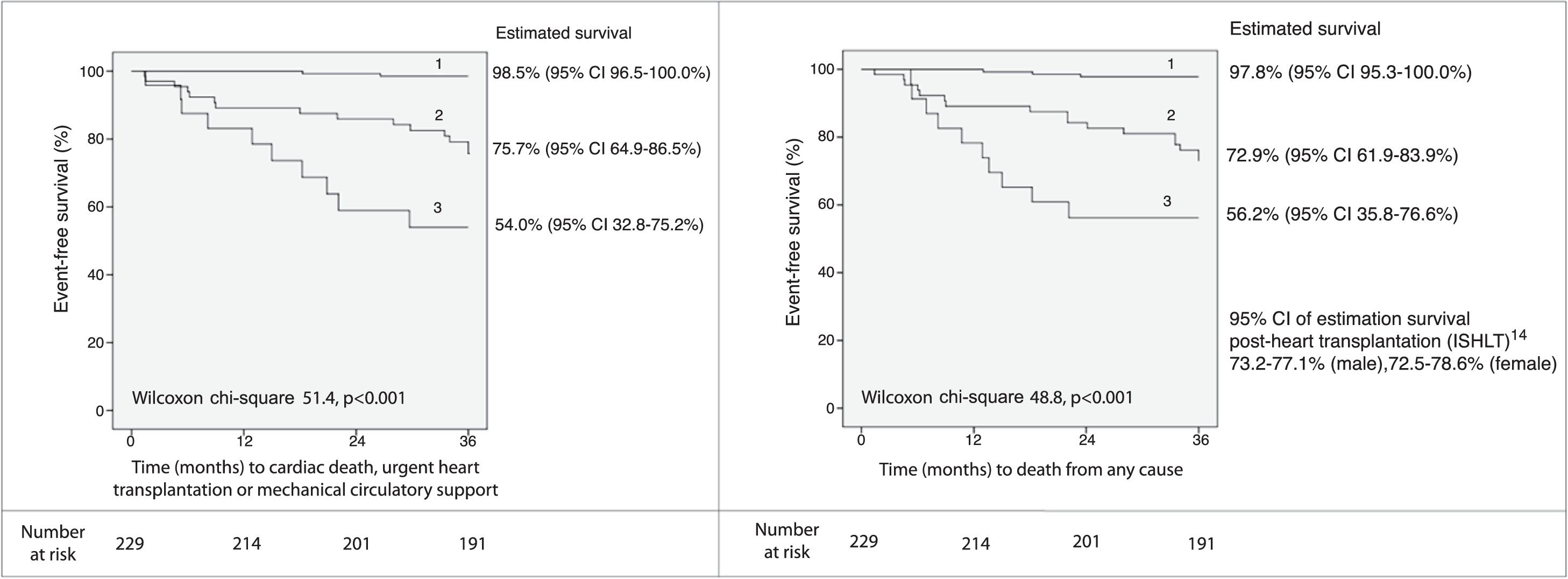
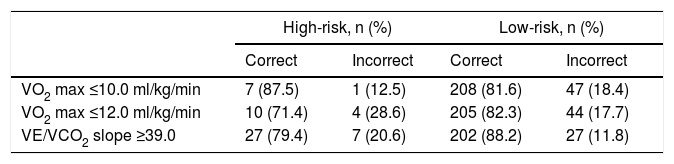
A threshold of 39.0 for VE/VCO2 slope was assessed as a potential listing criterion for HTX. Firstly, the 95% CIs of estimated survival (considering all-cause death) for high- and low-risk subgroups did not overlap with those of post-HTX reported by the ISHLT.15 Secondly, the VE/VCO2 slope had a higher AUC than VO2 max, considering both as continuous variables (AUC of VO2 max 0.79, 95% CI 0.72-0.87; DeLong test for comparison p=0.009). Moreover, there was a significant improvement in the percentage of correct classification using the VE/VCO2 slope threshold of 39.0, in comparison to discretized VO2 max (Table 4): considering the VO2 max threshold of 10.0 ml/kg/min, the overall NRI and IDI were 82.2% (95% CI 52.3-112.1%) and 0.278 (95% CI 0.182-0.373), respectively; considering the VO2 max threshold of 12.0 ml/kg/min, the overall NRI and IDI were 93.3% (95% CI 63.4-123.2%) and 0.226 (95% CI 0.141-0.311), respectively. Since the data consistently favored the use of VE/VCO2 slope as an indication for HTX, high-risk patients (VE/VCO2 slope ≥39.0) were excluded from subsequent analysis. In the other 229 patients, 27 (11.8%) events occurred during 36 months of follow-up. Sodium and creatinine levels, LVEF, and variation achieved in CPET (anaerobic threshold minus baseline) of the end-tidal carbon dioxide partial pressure (Δ PetCO2) were independent predictors of adverse events at 36 months of follow-up (Table 3). The AUC was similar for these predictors. For LVEF, the flatness of the martingale residuals smoother did not enable a suitable cut-off point to be identified that discriminated high- and low-risk patients (Supplementary Figure 2); sodium ≤136.0 mEq/l, creatinine ≥1.0 mg/dl and Δ PetCO2 ≤0.45 kPa (3.4 mmHg) were associated with higher risk of adverse events (specificity 63%, 61% and 63%, sensitivity 77%, 74% and 74%, respectively). Eight subgroups were created according to the three mentioned cut-off values (Supplementary Figure 3). Prognosis was similar for patients with up to one variable (sodium, creatinine or Δ PetCO2) with abnormal values (classified according to the thresholds identified); prognosis was similar for patients with two variables with abnormal values, and event-free survival was worst when the three variables were classified as abnormal. Three groups were created accordingly (Figure 2); prognosis was significantly different for the three categories.
Proportion of patients correctly and incorrectly classified at 36 months of follow-up.
| High-risk, n (%) | Low-risk, n (%) | |||
|---|---|---|---|---|
| Correct | Incorrect | Correct | Incorrect | |
| VO2 max ≤10.0 ml/kg/min | 7 (87.5) | 1 (12.5) | 208 (81.6) | 47 (18.4) |
| VO2 max ≤12.0 ml/kg/min | 10 (71.4) | 4 (28.6) | 205 (82.3) | 44 (17.7) |
| VE/VCO2 slope ≥39.0 | 27 (79.4) | 7 (20.6) | 202 (88.2) | 27 (11.8) |
VO2 max: peak oxygen consumption; VCO2: carbon dioxide production; VE: minute ventilation.
Event-free survival up to 36 months of follow-up (excluding patients with VE/VCO2 slope ≥39.0) and 95% CI of estimated survival for each subgroup, according to the combination of sodium (136.0 mEq/l), creatinine (1.0 mg/dl) and variation of end-tidal carbon dioxide partial pressure (0.45 kPa) cut-off values. Group 1: up to one abnormal parameter; group 2: two abnormal parameters; group 3: three abnormal parameters. CI: 95% confidence interval; ISHLT: International Society for Heart and Lung Transplantation; VCO2: carbon dioxide production; VE: minute ventilation.
The most accurate predictor of adverse outcome in patients with HF with reduced LVEF was VE/VCO2 slope and the best threshold for identifying patients who may benefit from HTX was 39.0. Sodium levels, creatinine levels and Δ PetCO2 were able to identify low-risk patients with excellent outcome.
Risk stratification in HF and selecting patients for HTX are challenging. Mancini et al.18 showed in an ancillary study that VO2 max is a valuable parameter for selecting patients for HTX, and subsequently refined their cut-off values.11,19 Currently, the American Heart Association recommends listing ambulatory patients for HTX when VO2 max is <10 ml/kg/min with achievement of anaerobic metabolism, and to defer when VO2 max is >14 ml/kg/min.4 Mancini et al.19 proposed a similar decision algorithm, also recommending assessment using the Heart Failure Survival Score (HFSS). The ISHLT recommends listing when VO2 max is <12 ml/kg/min on beta-blockers, deferring when it is >14 ml/kg/min, and using the HFSS in the gray zone.5 Only in the presence of a submaximal CPET should VE/VCO2 slope be considered as a listing criterion, according to the ISHLT guidelines.5 Risk stratification and patient selection for HTX could probably be improved.7 However, the use of randomized controlled trials to address the issue of listing criteria is hindered by ethical and social considerations. In this context, data from robust registries on non-transplanted HF patients receiving contemporary pharmacological and device therapy are of great value.
We evaluated an extensive range of clinical, laboratory, electrocardiographic, echocardiographic, and CPET parameters as potential predictors of adverse outcome. The single best parameter of all those studied was VE/VCO2 slope, yielding the highest Wald chi-square value and the highest AUC at 12, 36 and 60 months of follow-up, and with even more discriminative power than the HFSS, which combines different variables. Of note, VO2 max did not remain in any multivariate model in our cohort. The 60-month follow-up period was not used to identify an approach for selecting patients for HTX, since prognostic reassessment should be undertaken earlier.7,8 Nevertheless, the long-term follow-up carried out confirmed the consistently higher accuracy of VE/VCO2 slope over a long period, in comparison to other parameters. This finding has not been properly addressed in previous studies.6–8 Arena et al.6 showed that the risk of adverse events increases continuously over different categories of VE/VCO2 slope. However, thresholds are useful for clinical practice, and VE/VCO2 slope values of 34.0 and 35.0 have been proposed as optimal criteria for classifying patients with HF as high- and low-risk.5,14 In line with these thresholds, the risk of adverse events began to rise for values above 32.0 in our cohort, as shown by analysis of the martingale residuals. Although the specificity for this threshold was not low (80%), a non-negligible proportion of patients would be classified as high-risk even though they would not experience an adverse outcome. If this was considered a listing criterion for HTX, issues related to the shortage of donors and to the morbidity and mortality following HTX might arise, by listing patients who were at a less severe stage of HF. Therefore, the threshold of 39.0, which provides very high specificity with reasonable sensitivity, may be more appropriate for selecting patients for HTX than lower cut-off values.5,14 Freedom from the combined endpoint at 36 months of follow-up was very low (19.3%) for patients with VE/VCO2 slope ≥39.0. Even though this threshold was not primarily identified to predict total mortality, the 95% CI of estimated overall survival of high- and low-risk subgroups did not overlap with those of post-HTX reported by the ISHLT.15 This finding suggests that, for our cohort, survival of hypothetically transplanted patients would be better than survival of non-transplanted high-risk patients and worse than survival of non-transplanted low-risk patients. In addition, the threshold of 39.0 for VE/VCO2 slope was more accurate than the cut-off values of 10 or 12 ml/kg/min for VO2 max, which are recommended as listing criteria.4,5 VE/VCO2 slope has previously been reported as providing a discriminative power at least as good as VO2 max for predicting adverse events.6,9 Nevertheless, the majority of studies that highlighted the value of VE/VCO2 slope did not assess this parameter in the light of a comprehensive assessment of clinical, laboratory, electrocardiographic, echocardiographic and CPET parameters, and few had a long-term follow-up.6–8 Compared to previous studies, we carried out a more comprehensive (and prospective) baseline assessment, with a long-term follow-up, and employing a robust statistical analysis with consistent results. Based on our results, patients with VE/VCO2 slope ≥39.0 may benefit from HTX.
For low-risk non-transplanted patients with sodium levels >136.0 mEq/l, creatinine levels <1.0 mg/dl and Δ PetCO2 >0.45 kPa (3.4 mmHg), or patients with up to one of these variables classified as abnormal, the prognosis was excellent and total mortality was lower than that reported for post-HTX.15 Sodium and creatinine levels are known independent predictors of adverse events in HF and are included in risk scores such as the HFSS and the Meta-Analysis Global Group in Chronic Heart Failure score.1,2 PetCO2 at rest and at the anaerobic threshold were shown to stratify risk beyond the VE/VCO2 slope, and combining them into a single parameter (Δ PetCO2) may be more practical and accurate.20,21 We suggest that particular attention should be paid to sodium levels, creatinine levels and Δ PetCO2, in addition to VE/VCO2 slope, particularly for identifying low-risk patients in clinical practice.
Some limitations of the study should be acknowledged. Firstly, the analyzed cohort was not large. However, it was possible to identify the most important independent predictors of adverse outcome in HF and a strategy for optimizing the selection of patients for HTX, and the results were consistent using different statistical analyses. The sample size is similar to those of other studies that highlighted the value of CPET parameters, including for the selection of patients for HTX.6,14,18,20,21 Secondly, this was a single-center study. Nevertheless, this meant that the CPET protocol was consistent in all cases, and may have reduced the number of physicians responsible for the interpretation of the exam, reducing interobserver variability. Thirdly, listing for HTX is a complex and multidisciplinary decision and should not rely solely on ‘magic numbers’ of specific parameters from complementary exams; however, thresholds are useful in clinical practice, as pointed out above. In addition, the aim was not to replace but to potentially optimize current listing criteria for HTX. The decision threshold we propose is in line with current practice in different centers, where clinical decisions are supported by VE/VCO2 slope data in addition to VO2 max, even though current guidelines do not address this approach.4,5,7 A further validation study would certainly be useful.
ConclusionsAmong a large variety of predictors of adverse outcome in ambulatory patients with HF with reduced LVEF, the most accurate was VE/VCO2 slope. Patients with VE/VCO2 slope of 39.0 or higher may benefit from HTX. Beyond the VE/VCO2 slope, sodium levels, creatinine levels and Δ PetCO2 were able to identify patients with excellent outcome.
FundingNone.
Conflicts of interestThe authors have no conflicts of interest to declare.


 ISHLT: International Society for Heart and Lung Transplantation; VCO2: carbon dioxide production; VE: minute ventilation.' title='Event-free survival up to 36 months of follow-up according to VE/VCO2 slope threshold of 39.0, and 95% CI of estimated survival for each subgroup. CI: 95% confidence interval;
ISHLT: International Society for Heart and Lung Transplantation; VCO2: carbon dioxide production; VE: minute ventilation.' title='Event-free survival up to 36 months of follow-up according to VE/VCO2 slope threshold of 39.0, and 95% CI of estimated survival for each subgroup. CI: 95% confidence interval; 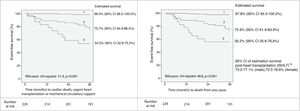 ISHLT: International Society for Heart and Lung Transplantation; VCO2: carbon dioxide production; VE: minute ventilation.' title='Event-free survival up to 36 months of follow-up (excluding patients with VE/VCO2 slope ≥39.0) and 95% CI of estimated survival for each subgroup, according to the combination of sodium (136.0 mEq/l), creatinine (1.0 mg/dl) and variation of end-tidal carbon dioxide partial pressure (0.45 kPa) cut-off values. Group 1: up to one abnormal parameter; group 2: two abnormal parameters; group 3: three abnormal parameters. CI: 95% confidence interval;
ISHLT: International Society for Heart and Lung Transplantation; VCO2: carbon dioxide production; VE: minute ventilation.' title='Event-free survival up to 36 months of follow-up (excluding patients with VE/VCO2 slope ≥39.0) and 95% CI of estimated survival for each subgroup, according to the combination of sodium (136.0 mEq/l), creatinine (1.0 mg/dl) and variation of end-tidal carbon dioxide partial pressure (0.45 kPa) cut-off values. Group 1: up to one abnormal parameter; group 2: two abnormal parameters; group 3: three abnormal parameters. CI: 95% confidence interval; 

