Heart failure (HF) with reduced ejection fraction (HFrEF) is associated with high rates of hospitalization and death. It also has a negative impact on patients’ functional capacity and quality of life, as well as on healthcare costs. In recent years, new HFrEF prognosis-modifying drugs have emerged, leading to intense debate within the international scientific community toward a paradigm shift for the management of HFrEF. In this article, we report the contribution of a Portuguese HF expert panel to the ongoing debate.
Based on the most recently published clinical evidence, and the panel members’ clinical judgment, three key principles are highlighted: (i) sacubitril/valsartan should be preferred as first-line therapy for HFrEF, instead of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker; (ii) the four foundation HFrEF drugs are the angiotensin receptor/neprilysin inhibitor, beta-adrenergic blocking agents, mineralocorticoid receptor antagonists, and sodium-glucose co-transporter 2 inhibitors, regardless of the presence of type-2 diabetes mellitus; (iii) these four HFrEF drug classes should be introduced over a short-term period of four to six weeks, guided by a safety protocol, followed by a dose up-titration period of 8 weeks.
A insuficiência cardíaca (IC) com fração de ejeção reduzida (ICFEr) está associada a níveis elevados de hospitalização e mortalidade. A ICFEr também tem um impacto negativo na capacidade funcional e na qualidade de vida dos doentes, bem como na despesa em saúde. Nos últimos anos, surgiram novos medicamentos modificadores do prognóstico da ICFEr, originando um intenso debate na comunidade científica internacional em relação a uma mudança de paradigma para o tratamento da ICFEr. Neste artigo, relatamos a contribuição de um painel de especialistas portugueses em IC para o debate em curso.
Com base na evidência clínica publicada mais recentemente e no julgamento clínico dos membros do painel, três princípios-chave são destacados: (i) sacubitril/valsartan deve ser preferido como terapia de primeira linha para a ICFEr, em vez de um inibidor da enzima de conversão da angiotensina ou um bloqueador do recetor da angiotensina; (ii) os quatro medicamentos básicos para a ICFEr são o inibidor do recetor da angiotensina e da neprilisina, os agentes bloqueadores beta-adrenérgicos, os antagonistas do recetor mineralocorticoide e os inibidores do cotransportador sódio-glucose 2, independentemente da presença de diabetes mellitus tipo 2; (iii) essas quatro classes de medicamentos para a ICFEr devem ser rapidamente introduzidas num período curto de 4-6 semanas, seguindo um protocolo de segurança, e depois tituladas durante as oito semanas seguintes.
Heart failure (HF) with reduced ejection fraction (HFrEF) is a fatal condition.1 In the late nineteen-eighties, 75% of HFrEF patients not receiving disease-modifying therapy would not survive beyond 5 years after the first symptoms.1 Additionally, HF is a major cause of hospitalizations and has a strong negative impact on patients’ symptoms, functional capacity and quality of life, as well as on health care costs.2 Hospitalizations are the main driver of HF-associated costs3 and are primarily caused by congestion.4
During the nineteen-sixties, thiazides5 and loop-diuretics6,7 were introduced to control congestion. By the end of the nineteen-eighties, vasodilators1 and angiotensin-converting enzyme inhibitors (ACEIs)8 had been proven capable of reducing HFrEF-related mortality. This was followed by an intense clinical development program, resulting in the emergence of angiotensin receptor blockers (ARBs),9 beta blockers (BBs),10 mineralocorticoid receptor antagonists (MRAs),11 angiotensin receptor/neprilysin inhibitors (ARNIs)12 and sodium–glucose cotransporter-2 inhibitors (SGLT2is),13,14 providing further significant reductions in HFrEF-related morbidity and mortality.
Although exciting, the present HFrEF therapeutic scenario has a complex decision tree in terms of which drugs to use first, in which patients, in what sequence to introduce the subsequent drugs, how fast to initiate and titrate them, and which safety monitoring protocol to use to avoid the most frequent serious side effects. A panel of six Portuguese HF experts convened to address these issues, based on the most recently published clinical evidence and on their own clinical judgment. This position paper contains three central messages:
- 1.
Sacubitril/valsartan (an ARNI) should be used instead of an ACEI or an ARB, as the preferential first-line therapy for HFrEF,15,16 unless it is not accessible or is not tolerated by the patient;
- 2.
The four foundational HFrEF drugs are ARNI (substituted by ACEI or ARB in patients with intolerance to ARNI), BB, MRA, and SGLT2i regardless of the presence of type-2 diabetes mellitus (T2D).17
- 3.
These four drug classes should be introduced at a low dose, over a short-term period of four to six weeks, followed by dose up-titration at a slower pace over the subsequent weeks18 and guided by a safety protocol.
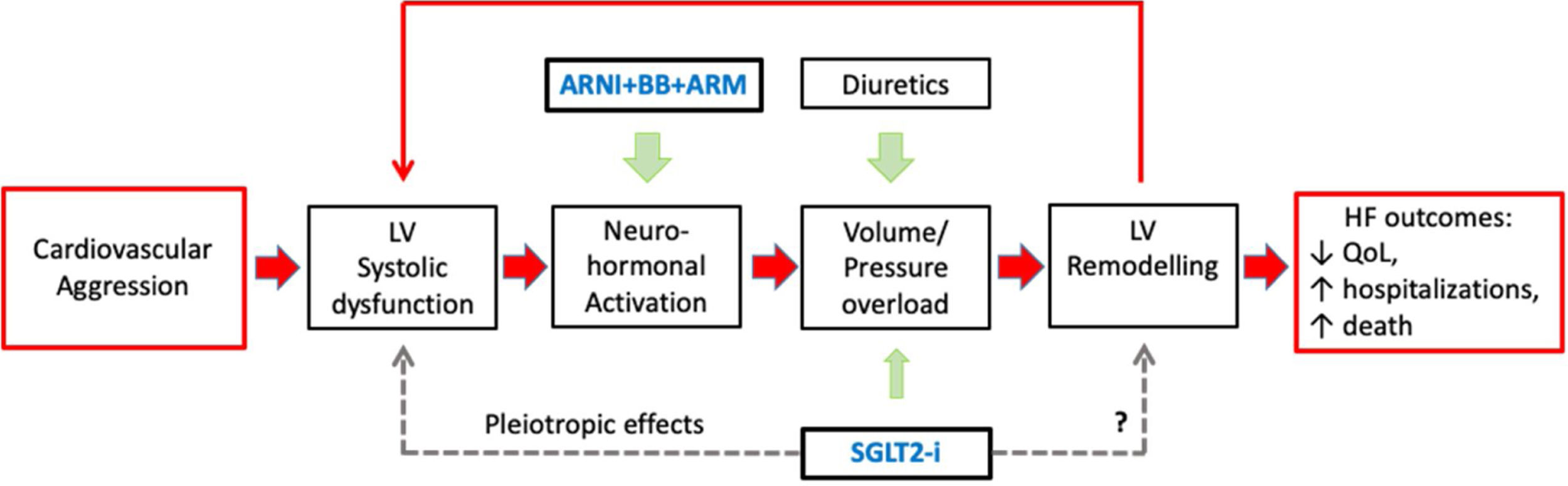
In the pathophysiology of HF, there is an important sequence of events: (i) the initial aggression that causes left ventricular (LV) dysfunction; (ii) neuro-hormonal (NH) activation; (iii) LV volume/pressure overload and remodeling; and (iv) further LV dysfunction and NH activation, in a vicious circle of inexorable HF worsening –‘the cardiomyopathy of overload’ – responsible for the HF poor prognosis.19 Hence the three central pharmacological strategies in HFrEF therapy are: congestion control, HR control, and reducing morbidity and mortality (see Figure 1).20
Central Figure. The pathophysiology of heart failure with reduced ejection fraction (HFrEF) and the four foundational HFrEF prognosis-modifying drugs: angiotensin receptor/neprilysin inhibitor (ARNI), beta-adrenergic blocking agents (BBs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose co-transporter 2 inhibitors (SGLT2i). The filled arrows represent recognized mechanisms; the dashed arrows represent possible mechanisms.
Diuretics are recommended to reduce the signs and symptoms of congestion, but their effects on mortality and morbidity have not been studied in randomized clinical trials (RCTs). A meta-analysis published in 2020 showed an association between loop diuretics and increased HF-hospitalizations and all-cause mortality,21 particularly when high diuretic doses were used. The authors suggested that prospective randomized studies could clarify whether this association is due to reverse causality.21 Diuretics therapy aims to achieve euvolemia with the minimal effective dose, as they stimulate the renin-angiotensin-aldosterone system (RAAS).20,22 However, because they reduce LV end-diastolic pressure, diuretics may also reduce cytoskeletal stimulation,23 which could have a positive effect on HF physiopathology,24 resulting in an improvement in HF prognosis.25 The dose of diuretics needs to be adjusted according to the patient's condition over time.20,22
Heart rate controlAdrenergic stimulation in HF increases the heart rate (HR).26 This is common in HFrEF and is associated with poor outcomes.27,28 When the patient has left ventricular ejection fraction (LVEF) ≤35%, is symptomatic, and is in sinus rhythm with resting HR ≥70 beats/min, despite maximum tolerated dose of a BB and under an ACEI (or ARB) and an MRA, sinus node If-channel inhibition with ivabradine should be considered.20 The use of ivabradine is associated with a reduced risk of HF hospitalization or cardiovascular death.29 The optimal resting ventricular rate in patients with atrial fibrillation (AF) and HF is still a matter of debate, but it is believed to be in the range of 60–100 bpm.20 Ivabradine has no place in AF HR-control as its effects are exerted by acting on the sinus node.30
Reducing morbidity and mortalityAfter the late nineteen-eighties, ACEIs,31 followed by BBs10 and MRAs,11 emerged as central players in HFrEF prognosis-modifying therapy. Since 2014, two new drug classes have joined this group: sacubitril-valsartan12 and two SGLT2i, empagliflozin and dapagliflozin.13 More recently, for patients with a recent acute HF (AHF) event, other new players have been added to the HFrEF treatment portfolio: vericiguat,32 omecamtiv mecarbil,33 and intravenous ferric carboxymaltose.34 ACEIs, ARBs, MRAs and ARNI are collectively designated as renin-angiotensin-aldosterone system inhibitors (RAASi).35 RAASi may induce hyperkalemia, frequently leading to RAASi down titration/withholding which, in turn, is associated with a worsening prognosis.36 New potassium-binders (patiromer and sodium zirconium cyclosilicate) allow serum K to be maintained within normal levels, enabling RAASi therapy.37 Additionally, in patients under MRAs, it has been shown that SGLT2i reduce the occurrence of moderate to severe hyperkalemia.38,39
2014, a paradigm shift: angiotensin receptor neprilysin inhibitor instead of angiotensin-converting enzyme inhibitor as a first-line heart failure with reduced ejection fraction therapyIn 2014, the PARADIGM-HF12 trial demonstrated that in patients with HFrEF, sacubitril/valsartan versus enalapril reduced the risk of HF hospitalization/cardiovascular mortality, sudden cardiac death, death due to progressive HF and total mortality.12 It has also been shown that sacubitril/valsartan reduces the rate of renal function decline in HFrEF when compared with enalapril.40 Sacubitril/valsartan was also superior to enalapril in terms of symptom improvement, functional capacity and quality of life.12 Both drugs showed a similar safety profile.12
The positive impact of sacubitril/valsartan on sudden cardiac death (SCD) in HFrEF patients should be highlighted because in the SOLVD trial, it was shown that ACEIs do not prevent SCD in this population.31 This is particularly relevant given that 50% of HF patients are New York Heart Association (NYHA) class II.41,42 NYHA class II patient mortality at 20 months after diagnosis ranges from 7% to 15%43 with 64% of these patients dying suddenly.42,44 This is contrary to the common belief that NYHA class II represents a stable condition. Of note, the superiority of sacubitril/valsartan over enalapril regarding SCD achieved statistical significance as early as one month after the start of therapy.45
In patients hospitalized due to decompensated chronic HF or new onset HF, with/without previous exposure to ACEI/ARB, the safety of initiating sacubitril-valsartan, after hemodynamic stabilization, was demonstrated in the PIONEER-HF40 and TRANSITION46 studies.
Based on all the above clinical evidence, the American College of Cardiology (ACC)47 and The National Institute for Health and Care Excellence (NICE)48 now recommend sacubitril/valsartan as a preferred first-line treatment instead of an ACEI/ARB.
2019-2020: ‘The new kids on the block’The DAPA-HF13 study in 2019 and EMPEROR-reduced14 study in 2020, showed that sodium-glucose co-transporter 2 inhibitors (SGLT2i) (dapagliflozin and empagliflozin respectively), combined with recommended HFrEF therapy further reduced the risk of HF-hospitalizations13,14 and cardiovascular mortality,13 regardless of the type 2 diabetes (T2D). The apparent divergence between these two studies in relation to the effect on cardiovascular mortality, in favor of dapagliflozin, has been interpreted by HF experts as being due to differences in study design, such as patient characteristics, sample size and follow-up times.49
In the DAPA-HF study, it was demonstrated that dapagliflozin has a very early positive impact on worsening HF/cardiovascular death, which reached statistical significance as early as 28 days after randomization.50 Similarly, in the EMPEROR-Reduced study, the benefit of empagliflozin in reducing the risk of worsening HF events was found to be statistically significant at 12 days after randomization.51 Moreover, both studies have also shown a clear benefit in terms of cardiovascular and renal protection.13,14
Based on this body of evidence SGLT2i (dapagliflozin and empagliflozin) are presently considered the fourth pillar of HFrEF prognosis-modifying therapy, regardless of the presence of diabetes, and on top of the triple neurohormonal modulation/blockade (ACEI/ARB/ARNI+BB+MRA).17,47
The SOLOIST-WHF study showed a positive prognostic impact of sotagliflozin (a SGTL2 and SGLT1 inhibitor) compared to placebo in patients with T2D and a recent worsening HF-event.52 Sotagliflozin, initiated before or shortly after hospital discharge, has been shown to reduce cardiovascular mortality, HF-hospitalizations and urgent hospital visits.52
The ‘fantastic four’The current profusion of HFrEF disease-modifying drugs calls for a strategy on how to optimize their coordinated use in clinical practice. By the end of 2020, John McMurray and Milton Packer had challenged the classical drug implementation strategy based on the principle of introducing and full-titrating one single prognosis-modifying drug at a time before adding another prognosis-modifying drug.18 These authors highlighted the four foundation drug classes to be introduced as soon as possible in the HFrEF therapeutic regimen: ARNI, BB, MRA and SGLT2i.18 Johann Bauersachs, in an allegory to the Marvel Comics’ superhero-team ‘The fantastic four’ used this term to designate these four HFrEF disease-modifying drug classes.53 Comprehensive HFrEF disease-modifying therapy (ARNI+BB+MRA+SGLT2i) is clearly superior to the conventional therapy (ACEI/ARB+BB) in terms of reducing cardiovascular death or HF-hospitalizations (OR=0.38; 95% CI 0.30–0.47).54,55 These four classes of drugs should, therefore, be considered the new standard of HFrEF therapy.53
Strategies for the implementation of HFrEF prognosis-modifying drugs after 2020The classical view: Titrate first, add laterThe classical HFrEF therapeutic strategy mimics the historical sequence in which disease-modifying drugs were tested in RCTs from the late eighties onward.20 These foundational RCTs thus constituted the backbone of this evidence-based strategy which, in turn, emulated the clinical protocols used in the research and development programs. Furthermore, this approach incorporated evidence showing that disease-modifying drugs are more effective at high-doses than at low doses.20 Therefore, therapy initiation with an ACEI/ARB plus a BB at low doses was recommended, followed by up-titration to maximal target doses.20 For patients remaining symptomatic and with LVEF ≤35% despite this therapeutic regimen, a second step followed, with the addition of a MRA at low dose, subsequently up-titrated to maximum doses.20 Finally, in patients still symptomatic and with LVEF ≤35% under ACEI/ARB+BB+MRA therapy, replacement of ACEI/ARB by an ARNI, titrated to maximal doses, was recommended by the 2016 European Society of Cardiology Heart Failure guidelines.20
However, the above strategy may no longer be appropriate, as the drugs that were first discovered are not necessarily the most effective ones.18 Additionally, this classical strategy results in a lengthy period for the full implementation of disease-modifying drugs, often taking several (typically six or more) months to be completed.56 This is relevant because, as previously mentioned, time does matter, as the impact on prognosis of disease-modifying drugs reaches statistical significance in a short-term period of a few weeks – so early as two weeks, as observed for the empagliflozin induced benefit.11,12,49,54,57 Furthermore, in RCTs as well as in everyday clinical practice, a high percentage of patients cannot reach the optimal drug target doses due to the occurrence of side effects such as hypotension, hyperkalemia, or renal insufficiency.56
The accelerated pathway: Add first, titrate laterIt has recently been shown that the initial combination of two disease-modifying drugs at low dose generates better outcomes than full up-titration of only one disease-modifying drug.18,58 This calls for the combination of several disease-modifying drugs at low dose at the start of HF therapy.18 Since much of the clinical benefit of these drugs is observed within a few weeks,11,12,49,54,57 some authors propose that the four classes of HF disease-modifying drugs should be initiated during a short-term period of 4 weeks.18 McMurray and Packer suggest initiating therapy with the combination of a BB and a SGLT2i, followed within one to two weeks by the addition of sacubitril/valsartan, and after a further one to two weeks, the addition of a MRA.18 After that, the dose up-titration of the drugs should follow at a slower pace.18
The strategy of McMurray and Packer has a strong rationale, as it targets several different pathophysiological steps in a fast sequence, although there is no randomized clinical trial to support this. In addition, since their strategy calls for starting all four disease-modifying drug classes within 4 weeks, one could argue that the suggested specific sequence of drug initiation may not be determinant in clinical practice.
The 2021 Update to the 2017 American College of Cardiology Expert Consensus for Heart Failure TreatmentThe 2021 update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment47 proposes an algorithm for HFrEF stage C therapy, starting with an ARNI (preferably) or an ACEI/ARB plus a BB, which should be up-titrated every two weeks to the maximum tolerated or target doses.47 An MRA and/or SGLT2i may be added, as well as ivabradine, diuretics and/or a hydralazine+isosorbide dinitrate combination, according to the patient's condition/phenotype.47 This ACC Expert Consensus suggests considering dose up-titration every two weeks in the case of MRA, ivabradine, and/or hydralazine+isosorbide dinitrate until the maximum tolerated or target dose is achieved.47
The 2021 Update to the Canadian Cardiovascular Society/Canadian Heart Failure Society Heart Failure GuidelinesThe 2021 update to the Canadian Cardiovascular Society/Canadian Heart Failure Society (CCS/CHFS) heart failure guidelines recognizes the combination of the four foundational HF disease-modifying drug classes as the standard therapy for the majority of HFrEF patients.59 This includes an ARNI (preferably to an ACEi/ARB), plus a BB, plus a MRA, plus a SGLT2i.59 Depending on the patient's clinical characteristics, other therapies may also be recommended, in order to improve prognosis, symptoms, congestion, or HR control, and/or address comorbidities.59
The 2021 European Society of Cardiology/Heart Failure Association Heart Failure Guidelines previewIn a joint ESC/Heart Failure Association (HFA) Session at ESC Heart Failure 2021 Congress (June 29, 2021), Theresa McDonagh and Marco Metra addressed the upcoming 2021 Heart Failure ESC Guidelines and the expected update to the HFrEF therapy algorithm. ACEI is recommended for patients with HFrEF to reduce the risk of HF hospitalizations and death (class I indication, level of evidence (LOE) A). Sacubitril/valsartan is recommended as a replacement for an ACEI in patients with HFrEF to reduce the risk of HF hospitalization and death (indication class I, LOE B). Concomitantly, rapid initiation of a BB, plus an MRA, plus a SGLT2i is also recommended as a first step ‘to reduce mortality for all patients’. Additionally, a personalized approach is suggested ‘to reduce hospitalization/mortality for selected patients’, including diuretics and other drug interventions according to the patient's profile.60
The Portuguese Heart Failure Expert PanelThis proposal of the Portuguese HF Expert Panel for a HFrEF drug initiation algorithm builds on the McMurray and Packer's paper,18 the 2021 update to the 2017 ACC HF Expert Consensus,47 the 2021 update to the CCS/CHFS Heart Failure Guidelines, and the 2021 ESC/HFA Heart Failure Guidelines preview. In addition, this proposal distinguishes different strategies for diabetic and non-diabetic patients and includes a safety monitoring protocol to ensure rapid treatment initiation and up-titration. Finally, this proposal recommends that HFrEF treatment optimization should be individualized according to the patient's condition and phenotype.59 This algorithm aims to provide practical guidance on how to proceed in regard to HFrEF prognosis-modifying drug optimization in everyday clinical practice.
The following key evidence-based principles were considered:
- •
in HFrEF patients, sacubitril/valsartan is clearly superior to ACEIs in terms of prognostic impact12;
- •
dapagliflozin or empagliflozin, on top of optimized HFrEF therapy (ACEI/ARB/ARNI+BB+MRA) further reduce the risk of HF-hospitalisations13,14 and cardiovascular mortality, regardless of the presence of diabetes.13
Thus, the core of HFrEF disease-modifying therapy includes four drug classes: ARNI, BB, MRA and SGLT2i.
The panel also considered the following evidence-based observations:
- •
Time matters when initiating HFrEF disease-modifying therapy, since ARNI,12 BBs,57 MRAs,11,61 and SGLT2i13,14 all show a positive prognostic impact as early as a few weeks after the initiation of therapy;
- •
The combined action of two disease-modifying drugs at low doses shows a higher prognostic impact than a high dose of a single drug18,58;
- •
In HFrEF patients, with or without diabetes, SGLT2i, on top of optimized HFrEF therapy, are associated with a better prognosis compared to placebo13,14;
- •
Although evidence for SGLT2i prognostic gains in HFrEF results from studies where these drugs were used on top of HF optimized therapy, the current guidelines of the American Diabetes Association recommend SGLT2i for T2D patients with HF, particularly those with LVEF <45%, as part of the glucose-lowering regimen, regardless of hemoglobin A1c level, and in addition to metformin and comprehensive lifestyle intervention.62
In summary, we recommend that the traditional start low and go slow HFrEF therapy initiation aphorism20 be changed to a start low and add fast strategy, which means starting the four foundational HF disease-modifying drug classes within a four to six week interval. This initial effort should be implemented at low drug doses. Dose up-titration follows thereafter, as quickly as possible, guided by an effective safety monitoring protocol.
Considering the above, the Portuguese HF Expert Panel recommends the following protocol for the drug initiation, drug up-titration and treatment monitoring of HFrEF patients.
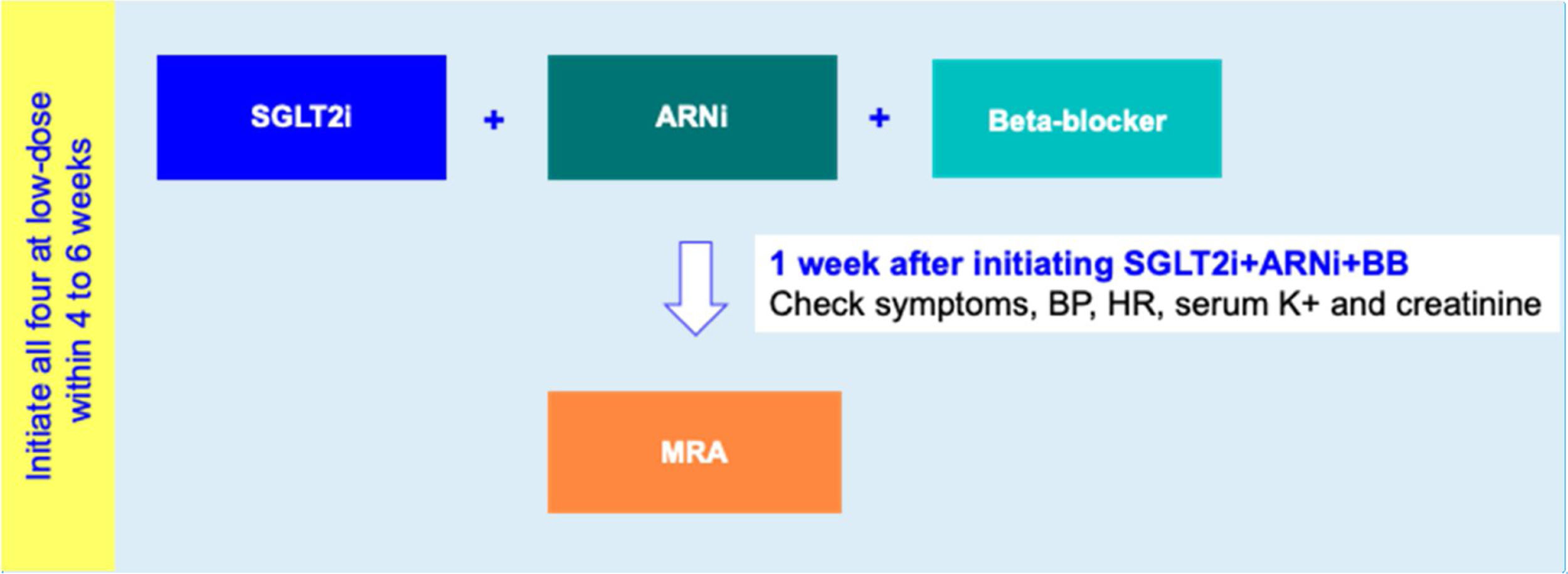
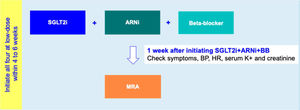
Drug initiation phaseFor HFrEF patients with T2D, a 2-step disease-modifying drug initiation strategy is recommended – see Figure 2:
- •
Step 1: Initiation of SGLT2i+ARNI (at an intermediate/low dose)+BB (at a low dose);
- •
Step 2: Addition of a MRA (at a low dose) 4 to 6 weeks after step 1.
Step 1 is in line with the 2021 guidelines of the American Diabetes Association which, as mentioned above, recommends timely SGLT2i therapy for T2D with HF.62
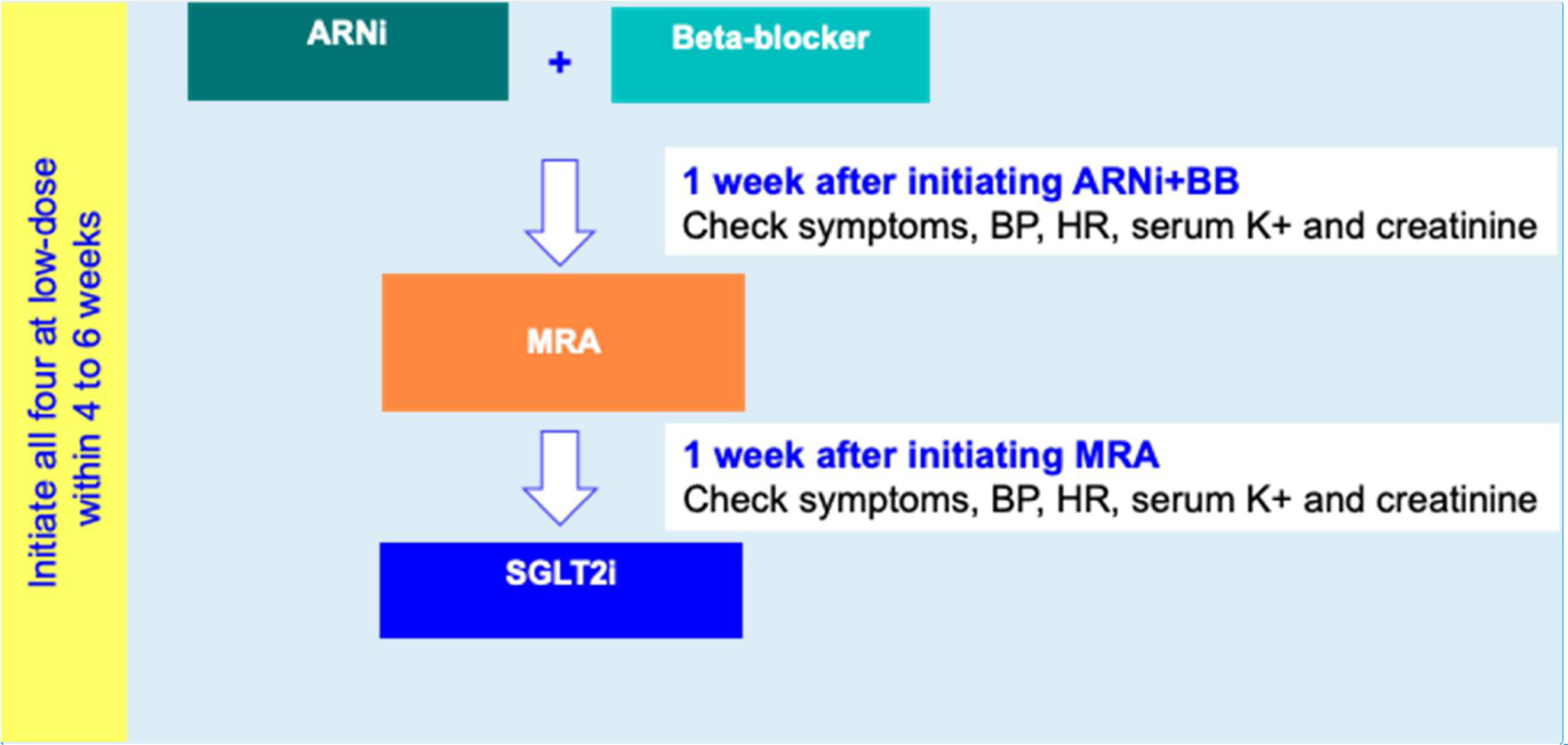
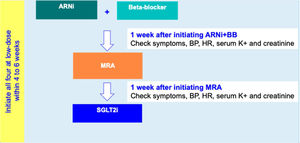
For HFrEF patients with no T2D, a three-step disease-modifying drug initiation with a SGLT2i on top of ARNI, BB and MRA, is recommended – see Figure 3:
- •
Step 1: Initiation of ARNI (at an intermediate/low dose)+BB (at a low dose);
- •
Step 2: Addition of a MRA (at a low dose) 2 to 3 weeks after step 1;
- •
step 3: Addition of a SGLT2i 2 to 3 weeks after step 2.
The four disease-modifying drug classes should be initiated within 4-6 weeks. In patients intolerant to ARNI, an ACEI or an ARB should be considered as an alternative.
Drug up-titration phaseIn HF, SGLT2i up-titration is not required.47 For the other three disease-modifying drug classes, we recommend up-titrating one drug at a time, to minimize the occurrence of complications. Up-titration steps for each drug should occur every two weeks at an in-person visit. Considering on average, one up-titrating step for ARNI, two for BBs and one for MRAs, most HFrEF patients may initiate and fully up-titrate all four disease-modifying drugs within a 12 to 14 week period.
Monitoring protocolWe suggest a monitoring protocol including in-person and remote appointments,63 to guarantee patient safety and allow a more agile therapy optimization.
During the drug initiation phase, we recommend the following:
- •
In step one, the in-person visit (day-1) Step one assigned drugs should be initiated and the patient should be instructed to record their daily symptoms, weight, HR and blood pressure. On day six, serum creatinine and potassium levels should be measured. On day seven, an online visit should be performed to check the above data and to modify the therapy, if needed for safety reasons.63
- •
The step two visit should be an in-person appointment, two to three weeks after the step one visit. The step two assigned drug should be initiated. The same methodology as described above for the step one visit should be followed.
- •
For non-diabetic HF patients, a step three in-person visit should take place two to three weeks after step two visit. The step three assigned drug should be initiated. The same methodology as described above for the step one visit should be followed.
During the drug up-titration phase, we recommend that each up-titration step occur at a two-week interval at an in-person visit. Patients should be instructed to record their daily symptoms, weight, HR and blood pressure. On day 6 after each up-titration step, measurement of serum creatinine and potassium levels should be performed. On day seven after each up-titration step, an online visit should be performed to check the above data and modify the therapy, if needed for safety reasons.
Telemonitoring may be useful during both initiation and up-titration phases especially in more unstable patients.63
In summary, the following three-step aphorism condenses the central messages of the Portuguese Heart Failure Expert Panel:
- •
start low (and early) and add fast;
- •
titrate later (to maximally tolerated doses);
- •
tailor according to the patient's profile (blood pressure, renal function, serum potassium level, HR and the presence of atrial fibrillation and/or other co-morbidities).
These recommendations of the Portuguese HF Expert Panel add to the ongoing international debate toward a HFrEF management paradigm shift. Our recommendations build on the central notion that presently, the core of HFrEF disease-modifying therapy includes four first-line drug classes: sacubitril/valsartan, beta-blockers, MRAs and sodium-glucose co-transporter-2 inhibitors. Sacubitril/valsartan, should be preferred as a first-line HFrEF therapy, instead of an ACEI or an ARB. In addition, our recommendations differentiate the implementation sequence of the above four drug classes according to HFrEF patients’ diabetic status and include a safety monitoring protocol to ensure rapid treatment initiation and up-titration.
Another important concept is that time is of essence when targeting better outcomes for HFrEF patients. Thus, ideally, all four drug classes should be introduced at a low dose during a four to six week period and up-titrated thereafter during a period of 8 weeks. Following this rationale, we suggest a safety monitoring protocol, including face-to-face and remote evaluations to facilitate rapid HFrEF treatment optimization.63 Telemonitoring should be implemented in more unstable patients.63
This set of recommendations by the Portuguese HF Expert Panel should not be regarded as a rigid protocol, but rather, as guidance, as HFrEF treatment optimization should always be adapted to the specific circumstances of the individual patient.
DisclaimersJ.S.C. has consulted and received speaker fees, or advisory board participation fees or investigational grants from Abbott, AstraZeneca Pharmaceuticals, Bial, Boehringer Ingelheim, Menarini, Merck Serono, Merck Sharp & Dohme, Novartis, Orion, Pfizer, Sanofi, Servier and Vifor Pharma. C.F. has received speaker and consultancy fees, advisory board participation fees, or investigational grants from Amgen AstraZeneca, Bayer, Bial, Boehringer Ingelheim, Merck Serono, Novartis, Orion, Pfizer, Roche Diagnostics, Servier, Vifor PHarma. F.F. has received speaker and consultancy fees or advisory board participation fees from AstraZeneca, Novartis, and Servier. J.M. has received speaker and consultancy fees, advisory board participation fees or investigational grants from AstraZeneca, Bayer Healthcare, Bial, Ferrer, Menarini, Merck Sharp and Dhome, Merck Portugal, Novartis, Pfizer/BMS, and Servier J.F. reports consultancy and lecture fees from Amgen, Astra-Zeneca, Boehringer Ingelheim and Novartis. D.B. has received speaker and consultancy fees, advisory board participation fees or investigational grants from Amgen, AstraZeneca Pharmaceuticals, Boehringer Ingelheim, Linde Saúde, Merck Portugal, Novartis, Orion, Pfizer, Roche Diagnostics, Servier, and Vifor Pharma.
Medical writer Duarte Oliveira (W4Research) collaborated in the preparation of this article, with financial support from Novartis Portugal. This article contains the authors’ opinion on the scientific contents addressed, which are expressed independently of Novartis. Novartis did not participate in the design, discussion of, or writing of this paper.