In the last 10 years the epidemiology of cancer has changed dramatically in Portugal, forcing the National Health System to revise its incidence predictions upward. In 2020 more than 55 000 new cases were identified and more than 65 000 are expected in 2035. All types of cancer are increasing, not only because of longer life expectancy, but also because of modern lifestyles and unhealthy habits. Since breast cancer is the second leading type of cancer and lymphoma is the ninth, and both types are treated effectively with anthracyclines, an increasing incidence of heart failure secondary to chemotherapy is to be expected in the near future.1 This, on top of the already high incidence of heart failure from other causes, is an issue of great concern in daily practice and in the management of the National Health System resources.2
In view of this problem, any attempt to reduce the incidence of chemotherapy-related cardiotoxicity with high cost-effectiveness is more than welcome. Unfortunately the evidence regarding cardio-oncology itself is scarce; most studies are observational and randomized trials have a small number of participants (in no case more than a few hundred), while randomized trials in oncology that have large study populations they report few cardiovascular side effects, which does not fit with real-world practice.3
In their study published in this issue of the Journal, considering these two issues – the need to reduce the burden of heart failure, and the lack of solid evidence in cardio-oncology – Sampayo et al. tested two heart failure treatment approaches, in order to determine which is more cost-effective.4 The conventional approach was extrapolated from the European Society of Cardiology cardiotoxicity guidelines,5 in which treatment with a beta-blocker and an angiotensin-converting enzyme inhibitor (ACEi) was started only after a diagnosis of heart failure, defined as an asymptomatic decline in left ventricular ejection fraction (LVEF) of ≥10% to a final value ≤55%. The second approach, universal cardioprotection (UCP), was derived from the OVERCOME trial,6 in which patients were randomized to enalapril and carvedilol (intervention arm) or no intervention (control arm).
Sampayo at al.4 used data from their own center to calculate the costs and quality-adjusted life-years (QALYs) of imaging-guided surveillance (cardioprotective drugs prescribed according to LVEF) or UCP (beta-blocker and ACEi for all patients). To obtain the QALYS and costs, they used a Monte Carlo simulation of a Markov model, the main characteristic of which is that although individual patients are subject to the same transition probabilities, they may or may not move between the stages of each cycle. Thus, the path followed by different patients will vary, due to random variability. In a Monte Carlo simulation, values are sampled at random from the input probability distributions. This process is computed hundreds or thousands of times, and the result is a probability distribution of possible outcomes.7 The microsimulation in the present study modeled a hypothetical cohort of 1000 patients over a five-year horizon. Although other base cases were tested, the major findings are obviously those of the reference case corresponding to a 63-year-old woman with breast cancer treated sequentially with anthracyclines and trastuzumab, for whom the LVEF-guided strategy (4.22 QALYs, €2594 over five years) was superior to the UCP strategy (3.42 QALYs, €3758 over five years). Although Markov models, like all statistical analyses, have their limitations, it is difficult to refute these findings, not only because patients are dissimilar in their genetic background, but also because the extent of the disease and comorbidities are different. This is why precision medicine is becoming so popular: one size does not fit all.
And why is precision medicine so desirable? First, depending on the series, cardiotoxicity occurs in 3-20% of cancer cases,8 so even in the worst scenario only one fifth of patients will have reduced LVEF; and second, management of neurohormonal blockade in cancer patients is generally more challenging than in the other major heart failure etiologies, especially because of hypotension resulting from vomiting, weight loss and co-medication. A UCP strategy is intuitively the worse option in these patients. Intuition apart, we should always be guided by evidence, and the most robust supports the treatment of reduced LVEF recommended in the guidelines,5 which is based on clinical trials with thousands of patients, from CONSENSUS in 1986 to the latest trials with sacubitril/valsartan and sodium/glucose cotransporter 2 inhibitors. These trials included many more participants than the two major studies of UCP in cancer patients, OVERCOME (90 patients)6 and PRADA (130 patients).9
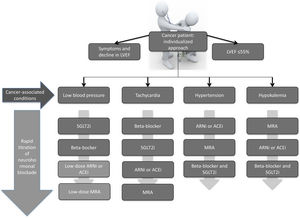
Interestingly, oncologists – like most physicians – believe strongly that cancer treatment should be started immediately after diagnosis, but unfortunately cardiologists are not aware that it is urgent not only to start heart failure treatment, but also to titrate it. This is why a personalized approach is so important in cancer patients with heart failure. The chosen therapeutics should be adjusted to the individual clinical presentation and comorbidities and should also be rapidly titrated (Fig. 1).
Different titration algorithms according to the clinical condition of cancer patients with heart failure. ACEi: angiotensin-converting enzyme inhibitor; ARNi: angiotensin receptor neprilysin inhibitor; LVEF: left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonist; SGLT2i: sodium-glucose cotransporter-2 inhibitor.
Nevertheless, cardio-oncology is definitely on the right track, as cardiovascular mortality in cancer patients has decreased significantly in recent years, as shown by the CARDIOTOX registry (0.4% cardiovascular mortality),10 much less than previous reports (16% cardiovascular mortality in breast cancer patients).11 The CARDIOTOX prospective registry included 865 patients and used LVEF to define cardiotoxicity. Myocardial dysfunction was identified in 37.5% of patients during follow-up (95% confidence interval 34.22-40.8%), 31.6% with mild, 2.8% with moderate, and 3.1% with severe myocardial damage/dysfunction. Despite the high incidence of cardiotoxicity, cardiovascular mortality was only 0.4%. It is our belief that these results are representative of contemporary cardio-oncology clinics, which follow the recommendations of the current ESC heart failure5 and European Society for Medical Oncology guidelines,12 treating patients only after an imaging diagnosis of myocardial dysfunction with an individualized drug approach.
Conflicts of interestThe author has no conflicts of interest to declare.