Cancer chemotherapy increases the risk of heart failure. This cost-effectiveness study analyzes cardio-oncology imaging assessment of left ventricular ejection fraction (LVEF) using a Portuguese healthcare payer perspective and a five-year time horizon.
MethodsTwo cardioprotective strategies were assessed: universal cardioprotection (UCP) for all patients and cardioprotection initiated on diagnosis of LVEF-defined cardiotoxicity (EF-CTX). A Markov model, informed by the retrospective clinical course of breast cancer patients followed in a Portuguese public hospital, was developed to assess the cost-effectiveness of LVEF cardio-oncology imaging assessment. Data on transition probabilities, costs and utilities were retrieved from both the retrospective data and published literature to assess the cost-effectiveness of LVEF echocardiographic assessment. Costs and utilities of the cardioprotective strategies were assessed over a five-year range, using probabilistic sensitivity analyses.
ResultsIn the reference case of a 63-year-old breast cancer patient treated with cardioprotection initiated on diagnosis of EF-CTX, the five-year time horizon (4.22 QALYs and €2594 cost over five years) dominated UCP (3.42 QALYS and €3758 cost over five years). Under a time horizon of five years at a willingness-to-pay threshold of €22 986, over 65.7% of simulations provided additional QALYs. Monte Carlo simulation of the Markov model had no effect on the model's conclusions.
ConclusionIn the Portuguese public healthcare system and under specific hypotheses, from a healthcare payer perspective, EF-CTX-guided cardioprotection for patients at risk of chemotherapy-related cardiotoxicity provides more QALYs at lower cost than UCP.
A quimioterapia aumenta o risco de insuficiência cardíaca. Este estudo de custo-efetividade analisa a avaliação da imagem cárdio-oncológica da fração de ejeção do ventrículo esquerdo (FEVE) usando a perspetiva do utente de serviços de saúde em Portugal e com um horizonte de tempo de cinco anos.
MétodosDuas estratégias de cardioproteção foram avaliadas: cardioproteção universal (UCP) para todos os doentes e cardioproteção iniciada no diagnóstico de cardiotoxicidade definida pela avaliação da FEVE (EF-CTX). Um modelo de Markov, informado pelo curso clínico retrospetivo de pacientes com cancro da mama seguidos num hospital público português, foi desenvolvido para avaliar o custo-efetividade da avaliação da imagem cárdio-oncológica da FEVE. Os dados sobre probabilidades transitórias, custos e utilidades foram obtidos dos dados retrospetivos e da literatura publicada para avaliar o custo-efetividade da avaliação ecogradiográfica da FEVE. Os custos e utilidades das estratégias cardioprotetoras foram avaliados num intervalo de cinco anos, usando a análise de sensibilidade probabilística.
ResultadosNo caso de uma paciente de 63 anos com cancro de mama, tratada com cardioproteção iniciada com avaliação da imagem da FEVE, num horizonte temporal de cinco anos (4,22 QALYS e custo de € 2.594 ao longo de cinco anos) dominou a cardioproteção universal (3,42 QALYS e € 3.758 ao longo de cinco anos). Em 65,7% das simulações, num horizonte temporal de cinco anos, e vontade de pagar até 22.986 €, a cardioproteção iniciada com avaliação clínica da FEVE acrescenta QALYs. A simulação de Monte Carlo do modelo de Markov não teve efeito nas conclusões do modelo.
ConclusãoNo sistema público de saúde português e com pressupostos específicos, do ponto de vista do utente de saúde, a cardioproteção definida pela avaliação da FEVE para pacientes com risco de cardiotoxicidade relacionada à quimioterapia fornece mais QALYs a um custo menor do que a cardioproteção universal.
Although anti-cancer targeted therapies lead to improvement in cancer survival, they also increase the incidence of cardiotoxicity, cardiac dysfunction and symptomatic heart failure.1–5 The current standard of care in Hospital Santa Maria, a public hospital in Lisbon, Portugal, involves regular monitoring of left ventricular ejection fraction (LVEF), with initiation of heart failure medications once LVEF falls to the point when a diagnosis is made of cardiotoxicity (conventionally defined as an asymptomatic fall in LVEF of ≥10% to a final value ≤55% or a symptomatic fall in LVEF of ≥5% to a final value ≤55%).6 This LVEF-guided definition of cardiotoxicity (EF-CTX) is a late stage of progressive myocardial functional impairment that begins at the time of the cardiac insult.7 An alternative strategy, universal cardioprotection (UCP), is based on a small randomized controlled trial of preemptive treatment of all patients with maximum tolerated doses of enalapril and carvedilol at the time of chemotherapy, which was demonstrated to reduce the incidence of cardiotoxicity and symptomatic heart failure compared with a control group.8 The disadvantage of this approach is that most treated patients do not develop EF-CTX or symptomatic heart failure and would have unnecessarily been exposed to the potential side effects and cost of medications.
No randomized trial has compared these options in the Portuguese context, so we developed a Markov model incorporating the probabilities and risks of the two cardioprotective strategies to determine the costs and quality-adjusted life-years (QALYs) obtained by each strategy in patients treated with potentially cardiotoxic chemotherapy.
The objective of this cost-effectiveness model is to analyze LVEF assessment using a healthcare payer perspective and a five-year time horizon. We used clinical data collected from cardio-oncology consultations in Hospital Santa Maria, in which the cardiology department has a cardio-oncology clinical specialty. Cardiac imaging exams are prescribed in the consultations, according to the malignancy and type of cancer. LVEF was used to measure cardiotoxicity in this cost-effectiveness analysis.
MethodsDataTo analyze the utility of cardiotoxicity monitoring, focusing on the impact of imaging-guided interventions, a comprehensive set of clinical data was required. Retrospective data including the clinical course of 109 patients from the first visit to the end of one year of treatment were collected from the cardio-oncology clinic of Hospital Santa Maria between November 2015 and November 2016. Patients in this study followed individual anti-cancer therapies and cardiac medication according to their cancer type. The patients’ mean age was 66 years. The mean age of the 51 patients with breast cancer was 63 years. A summary of the dataset is shown in Table 1.
Data summary.
| Variables | Breast cancer patients (n=51) | All patients (n=109) | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Variance | Min | Max | Mean | Variance | |
| Age, years | 40 | 86 | 62.80 | 147.84 | 19 | 97 | 65.94 | 191.45 |
| Number of visits | 2 | 3 | 2.22 | 0.17 | 1 | 3 | 2.18 | 0.15 |
| Heart failure risk score | 3 | 7 | 6.50 | 1.30 | 1 | 7 | 5.78 | 3.05 |
| Subsequent heart failure risk score | 1 | 5 | 2.19 | 1.38 | 1 | 6 | 2.19 | 1.38 |
| Heart rate, bpm | 47 | 120 | 80.24 | 182.14 | 47 | 120 | 78.50 | 208.97 |
| Gender | 100% female | 66% female, 34% male | ||||||
bpm: beats per minute; Max: maximum; Min: minimum.
This decision-analytic model assessed the morbidity, mortality, and costs associated with two strategies: the current standard strategy of initiating cardioprotective medications after diagnosis of EF-CTX (defined as an asymptomatic decline in LVEF of ≥10% to a final value ≤55%) or symptomatic heart failure; and a strategy of universal cardioprotection (UCP) for all patients at the time of chemotherapy. Cardioprotection was defined as concurrent enalapril and carvedilol up-titrated to their maximum dose, as used in the active treatment arm of a large recent randomized controlled trial,8 and used throughout the five years of modeling specialty. This Markov model used Monte Carlo simulations (Visual Basic for Applications in Excel) to assess the clinical and economic consequences of alternative cardioprotective strategies in a hypothetical cohort of 1000 patients in a microsimulation model.9 Beta distributions were assigned to probabilities and utilities, and gamma distributions to costs10 based on standard errors derived from our dataset and the literature. Cost-effectiveness acceptability curves (CEACs) were used to report the probability of incremental cost-effectiveness ratios (ICERs) at the willingness-to-pay threshold. The study was performed in accordance with the Consolidated Health Economic Evaluation Reporting Standards, as detailed by the International Society for Pharmacoeconomics and Outcomes Research.
Following Nolan et al.,11 the costs and QALYs of the treatments were estimated over a five-year period of the cohort, because transition probabilities beyond this period are not currently well described. It was assumed that all interventions took place at the start of the time horizon, and all future costs and benefits were discounted by 3% per annum. Given that probability data from the literature use a one-year cycle length, we transformed our three-monthly transition probabilities (in which the cycle length is four months) to a one-year cycle length.
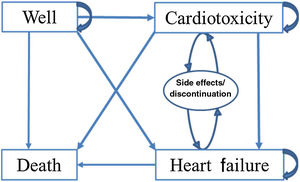
Markov modelThe Markov model accounted for the dynamics of cardiac screening and use of cardioprotective medications in a cohort of 1000 patients scheduled to receive chemotherapy for cancer. The base case is a 63-year-old woman with breast cancer receiving sequential anthracycline and trastuzumab therapy, which was applied to all cohort patients. This ‘average’ patient was assigned to one of the two treatment strategies. These patients progressed through the Markov model (Figure 1) on the basis of transition probabilities (Table 2), informed by utilities (Table 3) and costs (Table 4).
Transition states incorporated into the Markov model. In the Markov model patients begin in a state of no cardiac symptoms (i.e. well). During the next cycle, patients can remain in that state, die or be diagnosed as having cardiotoxicity or symptomatic heart failure. Patients can subsequently either remain in those states or die. Patients with cardiotoxicity may also be diagnosed with symptomatic heart failure before dying. Patients taking cardioprotective medications who can have side effects and/or discontinue the medication but do not leave the health state are represented by the oval.
Values for annual transition probabilities.
| Variables | Base value: breast cancer patients | Base value: all patients | Min | Max | Source | Distributiona |
|---|---|---|---|---|---|---|
| First year | ||||||
| LVEF-guided strategy | ||||||
| Cardiotoxicity | 0.128 | 0.198 | 0.050 | 0.300 | b | Beta |
| Heart failure | 0.000 | 0.000 | 0.010 | 0.20 | b | Beta |
| Death | 0.000 | 0.000 | 0.000 | 0.100 | b | Beta |
| UCP strategy | ||||||
| Cardiotoxicity | 0.1 | 0.1 | 0 | 0.35 | Nolan et al.11 | Beta |
| Heart failure | 0.03 | 0.03 | 0 | 0.05 | Nolan et al.11 | Beta |
| Death | 0.01 | 0.01 | 0 | 0.05 | Nolan et al.11 | Beta |
| Cardioprotective medication | ||||||
| Side effects | 0.330 | 0.330 | 0.250 | 0.640 | Nolan et al.11 | Beta |
| Discontinuation | 0.100 | 0.100 | 0.050 | 0.130 | Nolan et al.11 | Beta |
| Subsequent years (2-5) | ||||||
| Clinically well patients | ||||||
| Cardiotoxicity | 0.066 | 0.035 | 0.010 | 0.050 | b | Beta |
| Heart failure | 0.000 | 0.012 | 0.005 | 0.015 | b | Beta |
| Death | 0.000 | 0.046 | 0.001 | 0.009 | b | Beta |
| Patients with cardiotoxicity | ||||||
| Heart failure | 0.000 | 0.041 | 0.000 | 0.052 | b | Beta |
| Death | 0.000 | 0.041 | 0.000 | 0.008 | b | Beta |
| Cardioprotective medication | ||||||
| Side effects | 0.200 | 0.200 | 0.100 | 0.300 | Nolan et al.11 | Beta |
| Discontinuation | 0.050 | 0.050 | 0.020 | 0.080 | Nolan et al.11 | Beta |
Represents the probability derived from our dataset and confirmed by expert opinion; value ranges are from Nolan et al.11
Max: maximum; min: minimum; UCP: universal cardioprotection.
Annual costs in 2019 euros.
| Variable | Breast cancer patients | All patients | Source | Distributiona | ||||
|---|---|---|---|---|---|---|---|---|
| Base value | Min | Max | Base value | Min | Max | |||
| Hospital visit | 15.5 | 14.0 | 42.0 | 15.3 | 7.0 | 21.0 | DRE,14,b | Gamma |
| LVEF screening | 46.4 | 38.8 | 77.6 | 45.2 | 38.8 | 77.6 | DRE,14,b | Gamma |
| Cardiotoxicity | 1602 | 890 | 4450 | 2670 | 890 | 4450 | Nolan et al.11 | Gamma |
| Heart failure | 10 900 | 4450 | 17 800 | 10 900 | 4450 | 17 800 | 19 | Gamma |
| Cardioprotective medications | 48.04 | 4.97 | 305.94 | 64.7 | 5.0 | 335.1 | INFARMED,15,b | Gamma |
| Medication side effects | 667.5 | 44.5 | 4450 | 44.5 | 44.5 | 667.5 | Nolan et al.11 | Gamma |
Values for utilities.
| Variable | Breast cancer patients | All patients | Source | Distributiona | ||||
|---|---|---|---|---|---|---|---|---|
| Base value | Min | Max | Base value | Min | Max | |||
| Cardiotoxicity | 0.94 | 0.68 | 0.99 | 0.94 | 0.68 | 0.99 | Gohleret al.20 | Beta |
| Heart failure | 0.60 | 0.52 | 0.74 | 0.60 | 0.52 | 0.74 | Gohleret al.20 | Beta |
| Medication side effects | 0.96 | 0.92 | 1.00 | 0.96 | 0.92 | 1.00 | Lewis et al.21 | Beta |
| Medication side effects (discontinuation) | 0.99 | 0.95 | 1.00 | 0.99 | 0.95 | 1.00 | Imperiale et al.22 | Beta |
In the Markov model patients begin in a state of no cardiac symptoms (i.e. well). During the next cycle, patients can remain in that state, die or be diagnosed as having cardiotoxicity or symptomatic heart failure. Patients can subsequently either remain in those states or die. Patients with cardiotoxicity may also be diagnosed with symptomatic heart failure before dying. Patients taking cardioprotective medications may suffer from side effects and/or discontinue medication but do not leave the health state; these are represented by the oval in Figure 1.
Health states and transitionsData on transitions between health states were obtained from the dataset, the literature and expert sources (Table 2). From our dataset, transition probabilities were calculated from the proportion of patients in each state, following the procedure suggested by Black et al.12 and Miller and Homan.13 We closely followed Nolan et al.,11 who performed a similar cost-effectiveness analysis and gathered probabilities, cost and utilities from the literature. Transition probabilities were separated into two categories, initial year of treatment and subsequent years.
CostsThe economic analysis was from the healthcare payer's perspective and therefore used the amount reimbursed to the provider as the cost of care. Information regarding costs was obtained from our dataset and the literature (Table 3).
LVEF-guided echocardiographic screening costs were calculated by allocating the Portuguese official healthcare charges14 to the number of echocardiographic studies observed in the 109 patients. Calculation of medication costs included the medication indicated in the data, the active substance and dose, the price from the Portuguese National Authority for Medicines and Health Products (INFARMED) reference prices for generic medication,15 the government reimbursement rate and the number of packages needed for one year of treatment. Costs were expressed in 2019 euros and a willingness-to-pay threshold of €22 986 per QALY was applied, as this represents the 2019 average annual gross domestic product (GDP) of Portugal16 and a national per capita GDP value has been suggested by the World Health Organization to represent how much a nation can reasonably spend to save each QALY.17 Costs published in the literature more than two years earlier were corrected for intervening currency fluctuations using the Banco de Portugal's currency converter.18
Health outcomesTable 4 lists the utilities used in this study (values obtained from preferences associated with health-related quality of life where fully healthy=1.0 and dead=0.0). We followed Nolan et al.11 and the values were obtained from Gohler et al.,20 Lewis et al.,21 and Imperiale et al.22 Gohler et al.20 studied utility estimates for decision-analytic modeling in heart failure, with health states based on New York Heart Association classes and number of rehospitalizations. Lewis et al.21 assessed preferences for quality of life or survival expressed by patients with heart failure using the Minnesota Living with Heart Failure questionnaire. The patients’ utility for medication side effects also refers to patients with heart failure.
Scenario analysisTo determine the applicability to other types of cancer of the cost-effectiveness analysis of cardioprotective strategies, one additional scenario was run using the same Markov model. The scenario used the mean of all patients in our database, i.e. a 66-year-old patient with any type of cancer that involved chemotherapy treatment. This scenario includes patients with common cancer types, such as breast cancer, colon and rectal cancer, gastric cancer, kidney cancer, leukemia, lung cancer, melanoma, non-Hodgkin lymphoma and prostate cancer. Transition probabilities shown in Table 2 reflect the higher incidences of cardiovascular complications due to more aggressive chemotherapeutic regimens for cancers like Hodgkin lymphoma and acute myeloid leukemia.
Probabilistic sensitivity analysisProbabilistic sensitivity analysis was performed by varying all variables simultaneously over their plausible ranges. This process involved assigning distributions to 25 variables (15 event probabilities, six costs, and four utilities) used in the model. For the Monte Carlo simulation, 1000 iterations were run for both strategies using unique combinations of the 25 distributions, with values for each variable selected randomly. The differences between the strategies for each run were recorded to determine how often each was considered more cost-effective.
In probabilistic sensitivity analysis, a range of values reported from our dataset and found in the literature for transition probabilities, costs and utilities, were considered. When data were available, low and high values were chosen to reflect ranges in the literature. The model structure was based in part on other models in the literature, and was reviewed by clinicians involved in the care of cancer patients and chemotherapy-related cardiotoxicity.
ResultsHealth outcomes and costsIn the reference case of a 66-year-old patient taking anthracycline and trastuzumab for breast cancer, the outcomes of an LVEF-guided strategy (4.22 QALYs, €2594 over five years) were superior to those of UCP (3.42 QALYs, €3758 over five years). For a 66-year-old patient with any type of cancer, the outcomes of an LVEF-guided strategy (3.76 QALYs, €6718 over five years) were also superior to those of a UCP strategy (2.97 QALYs, €7995 over five years).
In Table 5, it can be seen that the findings for all patients are broadly similar to those of the base case, with the ICER for LVEF-guided strategy being negative for both, around 1.5 and 1.6, i.e. an LVEF-guided strategy is less expensive and more efficacious than UCP. However, the QALYs gained are smaller and the costs higher for all cancer patients compared to breast cancer patients, under both strategies.
Deterministic cost-effectiveness acceptability results.
| Scenario | Strategy | Cost in euros | QALYs | Cost per QALY in euros | ICER |
|---|---|---|---|---|---|
| Breast cancer patients | LVEF-guided | 2594 | 4.22 | 614 | -1458 |
| UCP | 3758 | 3.42 | 1097 | ||
| All patients | LVEF-guided | 6718 | 3.76 | 1787 | -1610 |
| UCP | 7995 | 2.97 | 2695 |
ICER: incremental cost-effectiveness ratio; LVEF: left ventricular ejection fraction; QALYs: quality-adjusted life-years; UCP: universal cardioprotection.
The effects of parameter uncertainty on the frequency of transition probabilities, utilities and costs were examined using a Monte Carlo analysis with 1000 simulations. This demonstrated that LVEF guidance was the optimum strategy for breast cancer patients, with a mean survival of 3.96 QALYS vs. 3.17 years with UCP (Table 6).
Probabilistic cost-effectiveness acceptability results.
| Scenario | Strategy | Average cost in euros | Average QALYS | Cost per QALY in euros | Probability of additional QALYs at willingness-to-pay | ||
|---|---|---|---|---|---|---|---|
| €5000 | €22 986 | €50 000 | |||||
| Breast cancer patients | LVEF-guided | 3486 | 3.96 | 881 | 61.4% | 65.7% | 65.6% |
| UCP | 4191 | 3.17 | 1322 | ||||
| All patients | LVEF-guided | 6896 | 3.79 | 3158 | 57.3% | 61.9% | 62.5% |
| UCP | 6966 | 3.06 | 3314 | ||||
LVEF: left ventricular ejection fraction; QALYs: quality-adjusted life-years; UCP: universal cardioprotection.
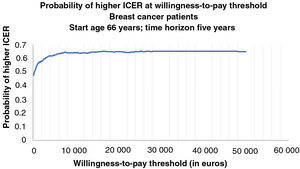
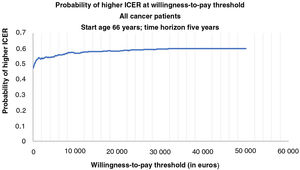
Figures 2 and 3 show the cost-effectiveness acceptability curves representing the probability that an LVEF-guided strategy is cost-effective for a given maximum willingness-to-pay threshold per QALY gained. The graphs are based on 1000 Monte Carlo simulations, drawing parameters for each input from probability distributions. Both cost-effectiveness acceptability curves demonstrated that an LVEF-guided strategy was consistently cost-effective across a broad plausible range of willingness-to-pay thresholds (from €0 to €50 000 per annum).
Cost-effectiveness acceptability curve for breast cancer patients representing the probability that each treatment strategy is cost-effective for a given maximum willingness-to-pay threshold per quality-adjusted life-year gained. The graph is based on 1000 Monte Carlo simulations, taking parameters for each input from probability distributions.
Cost-effectiveness acceptability curve for all cancer patients representing the probability that each treatment strategy is cost-effective for a given maximum willingness-to-pay threshold per quality-adjusted life-year gained. The graph is based on 1000 Monte Carlo simulations, taking parameters for each input from probability distributions.
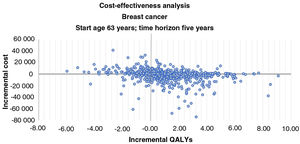
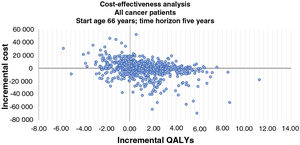
Incremental cost-effectiveness scatter plots of results of 1000 pairs of differences in costs and QALYs (Figures 4 and 5) show that an LVEF-guided strategy is more cost-effective than UCP. The comparison between LVEF and UCP shows clear benefit for LVEF for breast cancer patients, with LVEF being more cost-effective in 78.2% of instances, and for all patients, with LVEF being more cost-effective in 75% of instances.
Incremental cost-effectiveness bootstrap scatter plot for breast cancer patients. The y-axis represents the difference in mean costs (€2019), and the x-axis represents the difference in mean quality-adjusted life-years (QALYs). In 78.2% of iterations a left ventricular ejection fraction (LVEF)-guided strategy provided additional QALYs. Iterations in which an LVEF-guided strategy was less expensive and more efficacious than universal cardioprotection (UCP) accounted for 42.0% of the 1000 iterations. Iterations for which an LVEF-guided strategy provides additional QALYs at additional cost accounted for 36.2%. Iterations for which an LVEF-guided strategy is more expensive and less efficacious than UCP accounted for 15.9%, and iterations for which an LVEF-guided strategy is less expensive and less efficacious than the comparator accounted 5.9%.
Incremental cost-effectiveness bootstrap scatter plot for all cancer patients. The y-axis represents the difference in mean costs (€2019), and the x-axis represents the difference in mean quality-adjusted life-years (QALYs). In 75.0% of iterations a left ventricular ejection fraction (LVEF)-guided strategy provided additional QALYs. Iterations in which an LVEF-guided strategy was less expensive and more efficacious than universal cardioprotection (UCP) accounted for 39.8% of the 1000 iterations. Iterations for which an LVEF-guided strategy provides additional QALYs at additional cost accounted for 35.2%. Iterations for which an LVEF-guided strategy is more expensive and less efficacious than UCP accounted for 17.3%, and iterations for which an LVEF-guided strategy is less expensive and less efficacious than the comparator accounted 7.7%.
In this analysis of the cost-effectiveness of two strategies for targeting cardioprotective medications for the prevention of chemotherapy-related cardiomyopathy, an LVEF-guided strategy provided additional QALYs at a lower cost than UCP. The LVEF-guided strategy also produced better five-year survival, and was the optimal strategy in the majority of Monte Carlo simulations for breast cancer patients. When the Markov model was applied to patients with common cancer types, such as breast cancer, colon and rectal cancer, gastric cancer, kidney cancer, leukemia, lung cancer, melanoma, non-Hodgkin lymphoma and prostate cancer, the cost-effectiveness analysis shown similar findings but with lower QALYs and higher costs than with breast cancer patients. This group includes patients with cancers that have higher annual mortality rates and higher incidences of cardiotoxicity and cardiac failure due to more aggressive chemotherapeutic regimens23 for cancers like non-Hodgkin lymphoma. These results suggest that there may be significant differences in the cost-effectiveness of strategies depending on the malignancy involved. The conclusions of the model were robust when tested with probabilistic sensitivity analyses.
The increased survival associated with an LVEF-guided strategy reflects the high mortality burden of heart failure. Early detection of LV dysfunction can lead to early implementation of cardioprotective interventions such as initiation of cardioprotective medication. Identification of patients at higher risk is a key strategy to reduce morbidity and mortality from cardiotoxicity. The need to monitor cardiotoxicity in cancer patients has led to a new interdisciplinary specialty, cardio-oncology.3 Patients who may need cardioprotective medication can start medical therapy earlier when cardiotoxicity in being monitored properly. LVEF assessment can help to better adjust the therapeutic approach to cancer.24–26
Guidelines and consensus documents on cardiotoxicity, in general, define cardiotoxicity as an asymptomatic fall in LVEF of ≥10% to a final value ≤55% or a symptomatic fall in LVEF of ≥5% to a final value ≤55%.27 The guidelines are not universally consensual and there is no official standard care adopted worldwide. Both European and US cardiovascular society guidelines recognize the need to monitor and manage cancer patients. Recently, the European Society for Medical Oncology (ESMO) published recommendations for monitoring and treatment strategies for patients undergoing chemotherapy.28 The ESMO consensus document suggests the incorporation of surveillance strategies in cancer survivors since this will help prevent the potential long-term cardiovascular morbidity and mortality associated with cancer treatments. These recommendations corroborate the results obtained in this study, in which the LVEF-guided strategy provided additional QALYs at a lower cost than UCP.
LimitationsThe strengths of this analysis include accounting for a spectrum of cardiotoxicity after chemotherapy, for changes in costs and quality of life due to cardioprotective treatment, and for changes in the incidence of conditions in the first and in subsequent years. However, the study has limitations with regard to the quality of data entered into the model. Value ranges were drawn from retrospective data from different studies involving different populations and time periods. Costs of medical care and conditions differ in different countries and in different medical contexts, such as outpatient and inpatient care, which may have affected the results of modeling. For Portugal, we used retrospective data on 109 patients provided by the cardio-oncology clinic of Hospital de Santa Maria.
The utilities and transition probabilities used for the calculation of QALYs of the patients under UCP were designed for a population with breast cancer.11 Every patient is different, and cancer patients are very different from classic heart failure patients, for whom a major risk factor is hypertension, and angiotensin-converting enzyme inhibitors and beta-blockers are better tolerated. After weight loss and vomiting, cancer patients have very low blood pressure, and neurohormonal blockade is difficult to start and titrate. The side effects of the drugs and hence quality of life will differ. Furthermore, because the doses of the drugs are lower, the price paid will also be lower. Thus, this study would benefit from the greater accuracy of data that a prospective study would afford.
We were able to allocate to each patient under analysis the costs of medications in Portugal and charges for hospital visits and LVEF electrocardiograph assessment, which makes our cost analysis more reliable. The number of hospital visits assumed was based on the number of imaging-guided interventions. If a patient had one imaging-guided intervention, there must have been at least two hospital visits; if two imaging-guided interventions, at least three hospital visits, and so on. However, the costs of cardiotoxicity and medication side effects were taken from studies on US populations. The costs incurred for heart failure used in this study came from the published literature on Portuguese patients. These data limitations were partially overcome by the probabilistic sensitivity analysis, which showed consistent results with its deterministic analysis.
It would be interesting to apply the methods of this study to identification of subclinical injury through global longitudinal strain and to test its cost-effectiveness. Screening with strain imaging may be applicable to other situations in which there is a risk of developing heart failure. The prevalence of heart failure in mainland Portugal is predicted to increase by 30% by 2035 and by 33% by 2060, compared to 2011,29 and treatments that reduce its incidence have the potential to deliver substantial cost savings.
ConclusionIn the Portuguese public healthcare system and under specific conditions, from a healthcare payer perspective, an LVEF-guided strategy for targeting cardioprotective medications for patients at risk of chemotherapy-related cardiotoxicity provides more QALYs at lower cost than UCP.
FundingThis study was supported by Fundação para a Ciência e Tecnologia, under grant UIDB/00315/2020. The funding body had no influence on the design of the study, the collection, analysis, and interpretation of data, or the writing of the manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.