Chronic heart failure (CHF) is a growing public health concern and diagnosis can be challenging, particularly in primary care. This study aims to estimate the budgetary impact of introducing N-terminal pro-B-type natriuretic peptide (NT-proBNP) for CHF diagnosis in a primary care setting from the perspective of the Portuguese health system.
MethodsA budget impact analysis was conducted over one-year from the patients’ first presentation. The standard of care (SoC) was compared to NT-proBNP at the point-of-care (PoC) or laboratory (Lab). A decision tree model was used to estimate the downstream costs associated with each of the three pathways.
ResultsAn estimated 81 012 patients were expected to present to primary care with new onset CHF symptoms. The use of NT-proBNP as a primary diagnostic tool is estimated to generate annualized savings of EUR 935 657 and EUR 2 982 443 in the Lab and PoC setting, respectively. Estimated cost savings were due to the need for fewer medical visits, hospitalizations and echocardiograms (ECHO). The Lab and PoC settings led to similar reductions in hospitalizations (14.4%) and ECHO (27%), but the reduction in medical visits was higher in the PoC setting (38% compared to 2.5%), resulting in higher savings compared to Lab.
ConclusionsUsing NT-proBNP for CHF diagnosis in primary care could result in considerable costs savings for the public health system in Portugal. This evidence might support health policy makers to reconsider the resource management and define a new strategy to mitigate the impact of CHF.
A insuficiência cardíaca crónica (ICC) é um problema de saúde pública crescente, mas o diagnóstico continua desafiante, nomeadamente nos cuidados de saúde primários. O objetivo deste estudo é avaliar e quantificar o impacto orçamental para o Serviço Nacional de Saúde (SNS) da introdução do NT-Probnp nos Cuidados de Saúde Primários para o diagnóstico de ICC.
MétodosFoi efetuada uma análise de impacto orçamental para estimar a potencial economia de custos da utilização do NT-Probnp na avaliação inicial do doente com sintomas sugestivos de ICC, com o horizonte de um ano. Foram comparados três braços: cuidado padrão (cp) sem NT-Probnp; a utilização do biomarcador em point-of-care (poc) ou laboratório (lab). Foi usado um modelo de árvore de decisão para estimar os custos associados a cada um dos cenários.
ResultadosFoi estimado um total de 81 012 doentes com apresentação sugestiva de ICC. A utilização do NT-Probnp como meio diagnóstico primário pela medicina geral e familiar mostrou-se economizadora de custos, seja efetuado em lab ou POC, gerando uma economia anual estimada de 935.657€ e 2.982.443€, respetivamente. A economização de custos é devida à redução de consultas médicas, internamentos e ecografia (eco). A utilização de NT-Probnp em lab ou poc gera reduções idênticas em internamentos (14,4%) e eco (27%), mas a redução de consultas médicas é superior em poc (38% comparado com 2,5%), justificando a maior economia de custos neste contexto.
ConclusãoA utilização de NT-probnp no diagnóstico de ICC nos Cuidados de Saúde Primários pode resultar numa redução de custos considerável para o SNS. Esta evidência pode suportar os decisores de política de saúde a reconsiderar a gestão de recursos e a definir uma nova estratégia para mitigar o impacto da doença.
It is estimated that more than 400 000 people in Portugal suffer from chronic heart failure (CHF),1 accounting for nearly 4700 deaths per year, or about 5% of all deaths in mainland Portugal.2 CHF is a clinical syndrome that results in the reduction of cardiac output and/or elevated intracardiac pressure, due to structural and/or functional abnormalities.3 The most common symptoms of CHF are dyspnea and fatigue, which are not specific to this disease, often leading to a delayed or missed diagnosis. Given the prevalence and clinical severity, it is no surprise that CHF presents a significant economic burden to the Portuguese National Health System (NHS). In 2014, total direct costs for CHF were nearly 299 million euros, 39% of which were attributed to hospitalizations.4 The burden of CHF is expected to increase over time, with projections of a growth in both prevalence1 and costs4 by roughly 30% through 2035-2036.
Primary care physicians face significant challenges to correctly diagnose CHF early in the disease course. According to the European Society of Cardiology (ESC) Guidelines,3 the current diagnosis algorithm begins with the clinical evaluation of a patient's signs and symptoms, clinical history, physical evaluation, and complementary tests, such as electrocardiogram (ECG), chest x-ray, and blood analysis. However, these tests have low specificity for CHF. General practitioners (GP) are often required to rely on intuition and judgement, monitoring the patient's symptoms until they decide it is the appropriate time to request an echocardiogram (ECHO).
Echocardiogram is currently the gold standard for diagnosing CHF. Although generally available in Portugal, it is a relatively costly procedure; testing a large portion of suspected patients can result in a high degree of unnecessary use of healthcare resource and spending on patients that do not have CHF.
Natriuretic peptides (NP) are established biomarkers for evaluating patients with suspected heart failure (HF). B type NP (BNP) and its derivative, N-terminal pro-BNP (NT-proBNP), are released by the ventricles in response to volume and pressure overload. ESC guidelines recommend NP as complementary tests for the differential diagnosis of CHF, before ECHO.5 NT-proBNP has a very high sensitivity (99%) for HF when using a 125ng/mL threshold, with results below this cut-off indicating that HF is very unlikely. Thus, the use of NT-proBNP is expected to increase diagnostic specificity, which translates clinically to a reduction in unnecessary ECHOs, without compromising sensitivity, i.e., number of true cases detected. Compared to BNP, NT-proBNP has the advantage of not being affected by newer drug classes such as neprilysin inhibitors, making it a preferable biomarker for routine clinical practice.6 However, in Portugal, NP testing is not reimbursed in the primary care setting.
ObjectivesThe objective of this study is to assess the potential budget impact and financial implications associated with adopting NT-proBNP in the diagnostic journey for suspected CHF patients in primary care, from the perspective of the NHS in Portugal.
MethodsA budget impact analysis was performed to estimate the potential cost savings of using NT-proBNP in the diagnostic work up for suspected CHF patients. The model was developed to test the hypothesis that using NT-proBNP in the diagnostic journey would yield more accurate clinical decisions, reducing unnecessary ECHO on patients without the disease (reducing false positives) and giving an earlier and correct diagnosis to patients with the disease (reducing false negatives), allowing CHF patients to start treatment sooner and ultimately obtain better outcomes.
The decision tree analytic model was developed in TreeAge Pro 2017 (TreeAge Software, Inc, Williamstown, MA, USA) to capture all costs of the patient journey over one-year (2019), beginning at the first symptoms presentation and following patients through diagnosis and treatment within that year. The decision tree method was chosen due to the short time horizon, the population-level focus, and the straightforward nature of the outcomes.7 The model was built on a cohort distribution approach, where the target population followed different pathways, according to the estimated probability of having the disease and experiencing a hospitalization, exiting the model as CHF was confirmed or excluded.
While the patient flow and costs for each strategy were estimated using a model developed in TreeAge, the total budget impact calculations for Portugal were performed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). This analysis follows the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Principles of Good Practice Guidelines.7
Two primary data sources were used. Epidemiological data was directly inputted from the Portuguese EPICA study dataset.8 EPICA was a cross-sectional observational study based on a sample of the adult population age 25+ from health center patients in mainland Portugal from 1998 to 2000. Second, a review of published literature was conducted to populate the remaining clinical and diagnostic parameters, with preference for local studies. Additionally, all clinical authors: 1) provided input and feedback on the model structure development, 2) informed about the local resource use profile, 3) validated estimates and agreed on assumptions when data was unavailable.
Population and epidemiologyThe study population was estimated by the annual incidence of patients aged 25 years or older in Portugal evaluated by their GP with new signs and symptoms suggestive of CHF. The incidence rate by age group was estimated by Gouveia et al. based on the DisMod method, incorporating EPICA prevalence data.2 As more recent estimates were not available, epidemiologic data was updated accounting for changes in the demographic composition of the population in mainland Portugal in 2019, per recent data published by the National Institute of Statistics (INE).9
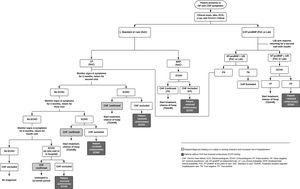
Model structureThe model decision tree was based on current clinical guidelines published by the ESC, along with the local clinical practice in Portugal. Three scenarios were compared in the analysis. First, in the current standard of care (SoC) scenario, clinicians were assumed to rely on clinical judgment (patient history, risk factors, physical exam) to assess disease probability and request ECHO to confirm or exclude HF diagnosis. The SoC was compared to two scenarios where NT-proBNP was used according to the ESC algorithm, prior to ECHO evaluation, including when performed at an external lab facility (Lab) or performed at the health center with a point-of-care device (PoC).
The primary outcome was the estimated budget impact in terms of change in total costs for the NHS, including diagnosis (medical visits and exams), treatment and hospitalization related to decompensated CHF. The model only considered direct medical costs. A visual depiction of the decision tree analysis is shown in Figure 1.
In each of the three scenarios, once diagnosis was confirmed by ECHO, either as CHF with reduced (HFrEF) or preserved ejection fraction (HFpEF), the patient began treatment over the remaining of the one-year period. Also, following the ESC guidelines,3 it is assumed that HFrEF patients were treated with angiotensin-converting-enzyme inhibitors (ACEi) and betablockers (βB), whereas patients with HFpEF were treated with diuretics. Treatment costs for comorbidities, e.g., hypertension or coronary artery disease, were not considered. The time to return with test results from NT-proBNP (external lab) and ECHO was assumed to be 15 days after the test was ordered (per expert's opinion).
Scenario one: standard of care – without NT-proBNPUpon entering the model, all patients with signs/symptoms suggestive of CHF underwent a complete clinical and physical evaluation by the GP, including a request for lab tests, electrocardiogram (ECG) and chest X-ray. ECHO was requested at the initial visit if probability of disease is estimated by GP as moderate/high (MHP). For modeling purposes, MHP was defined as a Boston Criteria for Diagnosing Heart Failure10 score I+II≥5 points (e.g. rest dyspnea and paroxysmal nocturnal dyspnea). We assume 35.8% meet this criterion according to EPICA data. Upon ECHO results, patients exited the model as true positives (confirmed CHF starting treatment) or false positives (unnecessary ECHO).
Patients who presented with milder and unspecific signs/symptoms of CHF (e.g. only dyspnea while climbing) were expected to have a longer diagnostic journey, given the nonspecific nature and frequency of such symptoms in the general population as well as time constraints and competing primary care issues.11 Thus, low probability (LP) patients, defined as Boston score I+II<5 points (64.2% of total patients per EPICA dataset), were considered to have ECHO requested subsequently to Visit 1. Of these, the majority (75%; [48.1% of total patients]) was assumed to have ECHO requested at Visit 2 (2 months). The remaining were expected to have ECHO requested at Visits 3 and 4 (5 and 11 months, respectively) using the same 75% split (12.3% and 1.3% of total patients, respectively), provided that no hospitalization due to decompensated CHF occurred at the end of the one-year period without treatment. For patients that were hospitalized at the end of the period, ECHO is assumed to be requested at the hospital admission where diagnosis was finally reached. Upon ECHO results, patients exited the model as described for MHP.
For MHP and LP, once CHF was confirmed by ECHO, patients were considered to begin and stay on treatment over the rest of the one-year period. Therefore, patients with ECHO requested at Visits 1, 2 or 3 were considered to receive treatment for 12, 10 and 7 months, respectively, while the remaining patients were assumed to stay untreated during the complete one-year period of the model.
Scenario two: with NT-proBNP at the point of careIn this scenario, in addition to the initial set of exams and clinical evaluation, NT-proBNP was also used as part of the initial assessment in the first visit for all patients with signs/symptoms of HF, in line with the ESC algorithm. Patients with a positive NT-proBNP test (≥125 pg/ml) had an ECHO requested during the same visit. After ECHO results, patients exited the model as true positives starting treatment as described above, or false positives. i.e. NT-proBNP+ECHO- (unnecessary ECHO). Subjects with a negative NT-proBNP test (<125 pg/ml) exited the model if they were true negatives, while false negatives followed the same monitoring approach as the LP group in the SoC arm.
Scenario three: with NT-proBNP at the labThe NT-proBNP Lab scenario was similar to the PoC decision tree, but included an additional FU visit to review the test results (as it was performed at an external facility), with an ECHO requested at that point. An estimated 15-day period was assumed between the exam request and receiving the results for either NT-proBNP and ECHO. Thus, treatment was considered to start one month after the initial visit. All remaining assumptions were the same as for NT-proBNP PoC.
Clinical dataThe clinical parameters evaluated in this analysis included the diagnostic test accuracy data for NT-proBNP, along with hospital and mortality data over the one-year time horizon of the model. The default values for each of these parameters are shown in Table 1.
Clinical and population inputs.
| Parameter | Default value | Reference/Assumption |
|---|---|---|
| Population inputs | ||
| Population size of mainland Portugal | 7 789 313 | INE Population projection, Portugal 20199 |
| CHF prevalence, projected to 2019 | 5.41% | Based on data from Fonseca 20181 |
| CHF incidence, projected to 2019 | 0.45% | Based on data from Gouveia, 20192 |
| Newly suspected CHF cases projected to 2019 | 81 012 | Based on data from Fonseca, 200413, Ceia 20028, Fonseca 20181, EPICA study (Data on file) |
| Newly confirmed CHF projected to 2019 | 42 190 | |
| Proportion with HFpEF (vs. HFrEF) | 40% | EPICA Study (data on file) |
| Diagnostic test accuracy | ||
| NT-proBNP (125 pg/ml) | ||
| Sensitivity | 99% | Taylor 201812 |
| Specificity | 60% | |
| Clinical assessment/Boston Score distribution | ||
| MHP (Boston≥5) | 35.8% | EPICA study (data on file) |
| Confirmed cases among MHP | 58.3% | |
| LP (Boston<5) | 64.2% | |
| Confirmed cases among LP | 48.6% | |
| Hospitalization and mortality | ||
| Hospitalization rate | ||
| Untreated | 68.1% | Based on Mant 200914 |
| Treated ≥8 months; <6 months | 53.4%; 57.3% | Calculated with Bayes theorem considering data from Mant 200914 and adjusted based on Fonseca 20181 |
| Treated ≥8 months; <6 months (adjusted) | 26.7%; 28.7% | |
| Mortality | 18% | Cowie 200229 |
CHF: congestive heart failure; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; LP: low clinical probability of heart failure; LP: low clinical probability; MHP: moderate or high clinical probability of heart failure; MHP: moderate/high clinical probability; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
The primary measure of effectiveness for NT-proBNP was the rate of false positive/false negative results, which lead to unnecessary testing and delayed diagnosis/treatment, respectively. The false positive/false negative rate was determined using relevant sensitivity, specificity, and prevalence data from the published literature and calculated according to Bayes’ revision theorem.1,12 For the SoC scenario, the accuracy of clinical assessment for the LP and MHP subgroups was derived from the EPICA dataset, with estimates based on the Boston score and confirmed by ECHO.13 As ECHO is the gold standard for diagnosing CHF, it was assumed that all results from ECHO were accurate and used to confirm or exclude the diagnosis.
Hospitalizations and mortalityThere are two main assumptions regarding hospitalizations. First, by obtaining an earlier diagnosis and earlier treatment, these patients were assumed to be less likely to have a hospitalization than when untreated. The effect of diuretics was not considered for HFpEF patients. Thus, the likelihood of having a hospitalization within the one-year period was directly related to the time spent on treatment. Second, if no CHF diagnosis was made prior to the patient having a hospitalization, the assumption was made that the diagnosis occurred at the time of admission.
For the base case we assumed a constant treatment effect for hospitalization, with a relative risk of 0.645 and 0.681 for HFrEF patients that were treated vs. untreated at 6 and 12 months, respectively.14 To estimate the hospitalization rate in a 12 month period, a weighted hospitalization rate based on Bayes’ theorem was calculated, considering the probability of not having a hospitalization in the first six months, as the patient was undiagnosed in the first period, and the probability of a treated patient having a hospitalization in the second 6 months. Due to the lack of specific data, patients treated for at least 8 months were assumed to have the same benefit as someone treated for the entire 12-month horizon; patients treated for six to eight months were assumed to receive the same benefit as someone treated for six months (implemented as a weighted calculation with six months untreated and six months treated); and patients treated for less than six months were not assumed to observe a benefit and the 12 month untreated rate was assumed. A correction factor of 0.5 was applied to adjust to HF hospitalization rates observed in Portugal (Table 1).1
Further, it was assumed that patients who died would have been hospitalized prior to death and therefore, to avoid counting them twice, a separate treatment effect on mortality was not included as it is already included within the hospitalization rate, nor was there a separate cost added for terminal care to avoid potential double counting. Additionally, no cost was attributed to death due to lost productivity (absenteeism) given CHF is far more prevalent in the elderly population who are not expected to be economically active.1,15
Cost dataAll unit costs for diagnostic testing, consultations and hospitalizations (Diagnosis-Related Groups (DRG) 194 – heart failure) were extracted from Portuguese official/public sources.16–18 Hospitalization cost was based on the average of grades 1-2 assuming an average length of stay of 8-9 days.
The same cost for the NT-proBNP test (code 22578 – Natriuretic peptide – Type B) was applied to both the PoC and Lab settings, and according to the source methodology, this cost included the procedure performed by the healthcare professional.
The treatment cost was applied for the duration of patient treatment. Drug treatment assignment/market share was estimated based on 1) the expert panel opinion on the therapy of choice in clinical practice; 2) patient distribution from official drug monitoring platform from NHS19; and 3) recommended dosages from the ESC Guidelines,3 assuming the average dose when a range of dosages was presented. Only monotherapy formulations were considered, and therapy cost was weighted by the pharmacologic class administered to each patient group (HFpEF and HFrEF) and the market share of the main drugs within each therapy class. All unitary costs were collected from official data source corresponding to the reimbursement percentage of out-patient prices supported by the Portuguese NHS (Table 2).20
Cost inputs.
| Parameter | Cost (€) | Reference/Assumption16-20 |
|---|---|---|
| GP visit | 31.00 | Ordinance no. 207/2017, Article 15 |
| NT-proBNP PoC | 29.60 | Ordinance no. 254/2018, 22578 – Natriuretic peptide (Type B) |
| NT-proBNP Lab | 29.60 | |
| Echocardiogram | 40.70 | Table “MCDT convencionados 2016”, cod. 1530.4 |
| Hospitalization (decompensated HF) | 1430.07 | Ordinance no. 254/2018, cod.40315 |
| Complete clinical assessment | 50.02 | |
| Electrocardiogram | 6.50 | Ordinance no. 254/2018, cod.40301 |
| Chest x-ray | 5.00 | Ordinance no. 254/2018, cod.10405 |
| Complete blood count | 4.70 | Ordinance no. 254/2018, cod.24209 |
| Sodium and potassium | 1.50 | Ordinance no. 254/2018, cod.22271 |
| Glucose | 1.10 | Ordinance no. 254/2018, cod.22076 |
| Renal function (urea, creatinine) | 2.50 | Ordinance no. 254/2018, cod.22949; cod.21620 |
| Hepatic function (AST, ALT, total bilirubin, GGT) | 5.50 | Ordinance no. 254/2018, cod.21220; cod.21217; cod.21340; cod.22035 |
| Thyroid stimulating hormone | 3.90 | Ordinance no. 254/2018, cod.22253 |
| Lipid profile (HDL, LDL, total cholesterol) | 5.60 | Ordinance no. 254/2018, cod.21539, cod.21545, cod.21554 |
| Iron, serum ferritin | 6.10 | Ordinance no. 254/2018, cod.21900; cod.21895; |
| Transferrin | 3.10 | Ordinance no. 254/2018, cod.22907 |
| Respiratory functional test (spirometry)* | 22.60 | Ordinance no. 254/2018, cod.80010 |
| Monthly medication costs | ||
| Diuretics (HFpEF) | Informed database, weighed average calculation of commonly used medications | |
| First month (loading dose) | 2.45 | |
| Following months | 3.79 | |
| ACEia/ARBsb+BBc+diureticsd (HFrEF) | ||
| First month (loading dose) | 10.89 | |
| Following months | 16.04 | |
ACEi: angiotensin-converting enzyme inhibitors; ALT: alanine aminotransferase; ARB: angiotensin receptor blocker; AST: aspartate aminotransferase; Bβ: beta-blockers; GGT: Gamma-glutamyl Transferase; GP: general practitioner; HDL: high-density lipoprotein; HFrEF: heart failure with reduced ejection fraction; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; Lab: laboratory; LDL: light-density lipoprotein; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NT-proBNP PoC: NT-proBNP at point of care; NT-proBNP Lab: NT-proBNP at independent lab; PoC: point-of-care.
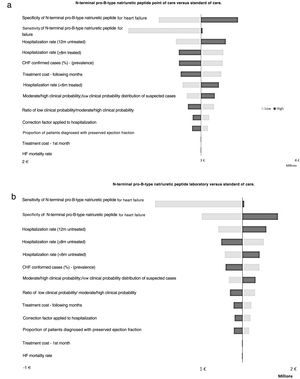
A one-way deterministic sensitivity analysis was conducted by varying different model parameters individually and observing the effect on the results in order to assess the impact of each variable and the robustness of the findings.21 The selected parameters and range over which the default values were varied can be seen in Table 3. The outcome tested was the incremental difference in costs for SoC vs. Lab and PoC.
Parameter ranges for sensitivity analysis.
| Parameter | Base case | Low | High | Notes |
|---|---|---|---|---|
| CHF confirmed cases (%) – (Prevalence) | 52.1% | 46.9% | 57.3% | ±10% of base case |
| Ratio of confirmed cases per LP/MHP | 59%/41% | 53%/47% | 65%/35% | ±10% of LP/MHP of confirmed cases from EPICA study |
| MHP/LP distribution of suspected cases | 35.8%/64.2% | 25.8%/74.2% | 45.8%/54.2% | ±10% of MHP/LP of suspected cases from EPICA study |
| Hospitalization rate (<6 months treated) | 57.3% | 51.6% | 63.1% | ±10% of calculated value |
| Hospitalization rate (≥8 months treated) | 53.4% | 48.1% | 58.7% | ±10% of calculated value |
| Hospitalization rate (12 months untreated) | 68.1% | 61.3% | 74.9% | ±10% of calculated value |
| Correction factor applied to hospitalization rate | 50% | 40% | 60% | ±10% of calculated value |
| HF mortality | 18.0% | 16.2% | 19.8% | ±10% of base case |
| Sensitivity of NT-proBNP for CHF | 99.0% | 57.0% | 100.0% | 95% CI (Taylor, 2019) |
| Specificity of NT-proBNP for CHF | 60.0% | 44.0% | 74.0% | 95% CI (Taylor, 2019) |
| Treatment cost – 1st month | 7.52€ | 5.64€ | 9.40€ | ±25% of calculated value |
| Treatment cost – Following months | 11.14€ | 8.35€ | 13.92€ | ±25% of calculated value |
| Patients diagnosed with HFpEF (%) | 40.0% | 25.0% | 60.0% | Expert panel opinion |
Each parameter was varied one at a time according to the range presented in the table to observe the impact on the model results.
CHF: congestive heart failure; CI: confidence interval; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; NT-proBNP: N-terminal pro-B-type natriuretic peptide; MHP: moderate/high clinical probability; LP: low clinical probability.
The estimated annual incidence of patients with newly suspected CHF in Portugal was 81 012, of which 42 190 were expected to be confirmed with either HFrEF or HFpEF. Regarding resource utilization, compared with the SoC, using NT-proBNP in a Lab setting was estimated to save 2.5% (5620) medical visits, 26.8% (21 137) ECHOs, and 14.4% (1890) hospitalizations. In the PoC setting, the reduction of ECHO and hospitalizations was similar, but more medical visits were avoided, 86 633 (38%), since NT-proBNP results were immediately available at the first visit.
Time to diagnosis was shortened in the NT-proBNP groups, with nearly all true CHF cases (99%) being diagnosed and starting treatment at one month for NT-proBNP groups, compared to 38.6%, 43.2%, 18.2% at one, two and after six months in the SoC, respectively. Furthermore, 100% of subjects without CHF had the disease correctly discarded at one month for NT-proBNP groups (60% NT-proBNP-; 40% NT-proBNP+ECHO-), compared to 32% in the SoC. As more patients have CHF ruled-in or ruled-out in earlier phases for NT-proBNP groups, fewer medical visits were overall needed compared to SoC.
Earlier treatment initiation in the NT-proBNP groups was associated with positive effects on hospitalizations and mortality, as more patients benefited from being more time on treatment. Importantly, the number of patients diagnosed with CHF at first hospitalization due to decompensation was estimated to fall from 5.2% in the SoC to less than 1% in NT-proBNP arms. The effect on mortality was also noticeable, with rates of 2.9% and 2.5% for SoC and NT-proBNP arms, respectively, representing 340 fewer deaths at one year.
Budget impact resultsThe total annual expected cost for the current SoC scenario was EUR 37 660 910 (Table 4). The introduction of NT-proBNP in the Lab setting was expected to save an estimated EUR 935 657 in annual costs, which is a 2.5% reduction in the estimated budget spent in the SoC scenario. When NT-proBNP was performed in PoC setting, it was expected to save an estimated EUR 2 982 443 in annual costs, or 7.9% of the SoC budget.
Cost savings of N-terminal pro-B-type natriuretic peptide (point of care and laboratory) versus standard of care.
| Cost Category | SoC | NT-proBNP PoC | NT-proBNP Lab | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cost | Total Cost | Savings vs SoC | Total Cost | Savings vs. SoC | ||||||
| Other Exams | 4 052 243€ | 11% | 4 052 202€ | 12% | 40€ | 0.0% | 4 052 202€ | 11% | 40€ | 0% |
| Visits | 7 001 046€ | 19% | 4 315 472€ | 12% | 2 685 574€ | 38.4% | 6 826 833€ | 19% | 174 213€ | 2.5% |
| NT-proBNP | 0€ | 0% | 2 397 944€ | 7% | -2 397 944€ | 100% | 2 397 944€ | 7% | -2 397 944€ | 100% |
| Echocardiograms | 3 207 127€ | 9% | 2 346 862€ | 7% | 860 266€ | 26.8% | 2 346 862€ | 6% | 860 299€ | 26.8% |
| Treatment | 4 595 413€ | 12% | 5 463 730€ | 16% | -868 317€ | -18.9% | 4 999 155€ | 14% | -403 742€ | -8.8% |
| Hospitalizations | 18 805 081€ | 50% | 16 102 256€ | 46% | 2 702 825€ | 14.4% | 16 102 256€ | 44% | 2 702 825€ | 14.4% |
| Total cost | 37 660 910€ | 100% | 34 678 467€ | 100% | 2 982 443€ | 7.9% | 36 725 253€ | 100% | 935 657€ | 2.5% |
Lab: laboratory; NT-proBNP: N-terminal pro-B-type natriuretic peptide; SoC: standard of care; PoC: point of care.
The Lab and PoC settings led to similar reductions of hospitalization costs (EUR 2 702 825) and ECHO (EUR 860 265), but the reduction of office visits related costs was higher in the PoC setting (EUR 2 685 574 compared to EUR 174 213), resulting in overall higher savings compared to Lab.
Sensitivity analysisThe results of the model were found to be robust to all changes in parameter values (Figure 2). The use of NT-proBNP retained savings in nearly all scenarios – the only exception being in Lab vs. SoC analysis, where the sensitivity of NT-proBNP for CHF was reduced to 57% (Table 3), causing an increment of 26.8 thousand euros. All other scenarios generated savings ranging from 489.6 thousand to 1.3 million euros in the Lab setting, and 2.2 million euros to 3.4 million euros in the PoC settings.
One-way sensitivity analysis results (tornado diagram - cost savings estimation).
The base case budget impact analysis yielded EUR 2 982 443 in savings in a point of care setting (a) and EUR 935 657 in the laboratory setting (b), with the bars on the tornado diagram representing the deviation from this base case when the default parameter value was changed. Positive values indicate costs that are avoided (cost savings).
CHF: congestive heart failure; HF: heart failure.
Overall, there was a much greater magnitude of change in the results (about three times greater) when varying parameter values in the Lab vs. PoC sensitivity analysis. This is because of the number of visits in correlation with epidemiological data, e.g., a 1% increase in the prevalence data corresponded to 810 more cases, which in turn corresponded to 810 additional visits to the Lab, compared to the PoC arm.
DiscussionChronic heart failure affects a significant portion of the population in Portugal and represents a huge and increasing burden for patients and the health care system. Medical technologies play a significant role in the patient care continuum, and health economics is becoming an indispensable element for valuing their implementation in clinical routine.
Diagnosis of CHF can be particularly challenging in primary care. The time from the first onset of symptoms to final diagnosis can range from a few months to years, with an average delay of nearly 10 months from symptom onset to diagnostic testing, and a total delay from symptoms to CHF treatment of over two years.11
There is vast evidence suggesting that using NT-proBNP can help identify and diagnose CHF patients earlier,12 but economic studies representing the Portuguese setting are missing.
Current guidelines recommend the use of NP for the differential diagnosis of patients with suggestive signs or symptoms of CHF.3 GPs in Portugal rely on subjective clinical judgment and interpretation, which can lead to variability and delayed diagnoses, yielding neither good sensitivity or specificity to consistently make a distinction between CHF and other possible causes for the patient's symptoms.
Our study findings suggest that including NT-proBNP for the differential diagnosis of CHF in primary care, according to ESC guidelines, would result in considerable cost savings for the system, whether performed in a Lab or PoC setting. These savings would be largely driven by allowing patients to be diagnosed and treated earlier leading to better patient outcomes. Compared to SoC, the NT-proBNP scenarios were associated with earlier rule-in and rule-out of CHF, resulting in a decrease in the burden of office visits and unnecessary ECHO, and, due to earlier treatment initiation, a reduction of avoidable hospitalizations and mortality.
Although the first of this type of analysis in Portugal, the findings of this study are in agreement with similar studies published in other countries and health systems showing a reduction of 25-31% in ECHO procedures and 13% in hospitalizations.14,22–28 In spite of the particularities of each health system structure and/or economic analysis methodology and parameters, all of them assessed NT-proBNP favorably in the primary care setting.
Importantly, NT-proBNP performed at the PoC is expected to result in a relevant reduction in the number of medical visits compared to the Lab, which generates substantial additional savings. PoC devices are widely available and are easy to use in a healthcare center, and therefore should be considered for better allocation of resources as our data suggest.
LimitationsThe model is a simulation of the CHF diagnostic journey in Portugal, which implies some simplification of the clinical pathway that is intended to apply to the majority of patients with suspected CHF. Furthermore, there are complexities and additional factors that may not have been considered in this model that apply to certain clinical scenarios and subpopulations.
Some conservative assumptions were made in this analysis that could potentially lead to greater cost savings. First, the analysis covers a short time period of only one year and as CHF is a chronic condition, the potential economic advantage of earlier diagnosis and treatment of CHF patients could go well beyond a one year by slowing down disease progression and avoiding costly downstream hospitalizations and procedures. Moreover, this analysis only includes the consequences of diagnosing and treating CHF patients earlier – thereby avoiding unnecessary ECHO testing and hospitalizations. However, greater cost savings and clinical benefits are expected when considering the pool of patients without CHF that would receive their proper diagnosis and treatment earlier. In addition, emergent treatment classes (angiotensin receptor neurolysin inhibitors and sodium-glucose transport protein 2) can lead to greater decreases in hospitalizations and mortality beyond that anticipated by our model. Aligned with the perspective of the NHS, this model only captures direct costs, and therefore does not account for indirect costs, such as lost productivity or impact on quality of life, for which there could also be a substantial benefit.
The main limitation of this analysis is that performance data for NT-proBNP are not available in the primary care setting in Portugal. We selected the 125 pg/mL cut-off value recommended by the ESC and used sensitivity/specificity data from a recent meta-analysis based on three studies in primary care with NT-proBNP.12 As different cut-offs may be used in other countries, this must be considered when comparing the results versus international literature.28 Also, the sensitivity/specificity data used refer to NT-proBNP from a specific manufacturer (Roche®), and therefore results are not directly transferable to other commercially available natriuretic peptides.
Several other variables were inputted from different sources that may introduce some bias to the model. However, the most critical variables – prevalence, distribution of visits, hospitalization rate, test sensitivity and specificity – were tested in different sensitivity analysis over a broad range of values that showed preserved savings in most scenarios, suggesting the results of the base case are robust.
ConclusionUsing NT-proBNP in primary care for CHF diagnosis could result in considerable costs savings and resource optimization for the national health system in Portugal. Given the significant impact of CHF, this evidence should be considered by health policy makers for reimbursement in primary care while planning a national HF strategy.
FundingThis study was supported by Roche Diagnostics. The authors had full control of the content and made the final decision on all aspects of this article.
Conflicts of interestCândida Fonseca received lecture and/or advisory fees from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Orion, Roche Diagnostics, Servier and Vifor Farma.
Paulo Bettencourt received lecture and/or advisory fees from Bial, Novartis, Roche Diagnostics e Servier.
Dulce Brito received lecture and/or advisory fees from Amgen, Amicus, AstraZeneca, Bayer, Boehringer Ingelheim, Linde Saúde, Merck, Novartis, Orion, Pfizer, Roche Diagnostics, Servier and Vifor Farma.
Helena Febra and Álvaro Pereira received lecture and/or advisory fees from Roche Diagnostics.
Nelson Lopes is employed by Roche Diagnostics.
We thank the EPICA investigators for allowing access to the study database.