| The ‘Velho do Restelo’ (Old Man of Restelo) was a personality created by the Portuguese poet Luís de Camões in scene IV of his epic work The Lusiads. Velho do Restelo symbolized the pessimists, the conservatives and the reactionaries who did not believe in the success of the epopee of the Portuguese discoveries and appeared at the departure of the first expedition to India (15th Century) with warnings about the odyssey that was about to occur… |
I may very well be seen as an Old Man of Restelo. In a series of editorials and interventions at congresses and other scientific meetings, in the last few years, I have manifested concerns about the fast spread of transcatheter aortic valve implantation (TAVI), but not about the concept and its role in the future, in which I believe. My main concern is that much of the enthusiasm about this novel technology may not be grounded in solid scientific proof. I concede that there is already important evidence that this procedure improves both the survival and quality of life for patients with severe symptomatic aortic stenosis who are not amenable to classical surgical aortic valve replacement (SAVR) or are deemed to be a too high risk for surgery, especially the elderly. In these circumstances, TAVI can be considered a ‘lifesaving’ procedure, given the dismal natural history of the disease, and nobody quarrels about this.
But the current guidelines developed by both European Associations of Cardiology and Cardiac Surgery, are quite clear about the indications for TAVI.1 The only Class I (level of evidence B) indication states that “TAVI is recommended in patients with severe symptomatic aortic stenosis who are not suitable for SAVR as assessed by a ‘heart team’ and who are likely to gain improvement in their quality of life and to have a life expectancy of more than one year after consideration of their comorbidities”. And the “too risky” situations already come as Class IIa indications. This is largely supported by the 2020 Guidelines of the American College of Cardiology/American Heart Association (ACC/AHA)2 which state that “the survival and symptom reduction benefit of TAVI is seen only in appropriately selected patients”. They further state that “patients with a mechanical impediment to SAVR, such as a porcelain aorta or prior chest radiation damage, may have better outcomes after TAVI than frail patients or those with moderate to severe disease in more than one other organ system”.
Hence, the problem takes on new dimensions when we hear about extending the indications to patients not belonging to those two categories – inoperable or too risky, whether the decision has been taken by a heart team or not. The same applies to extending the indication to other diseases, such as aortic regurgitation. One of the main issues is the durability of these valves, which is far from being proven. SAVR has been used for more than 50 years, with ample durability data available for specific valve types across different age groups. Currently, robust durability data for TAVI extend to only about five years. SAVR valve deterioration typically occurs after >10 years, so longer-term TAVI durability data are needed. Hence, one thing is using TAVI in patients with a short life expectancy and another one is using it in patients who naturally expect to live a reasonably normal length of life, which has long been granted by SAVR, with survival that approaches that of the normal matched population.
Such “off label” utilizations of the technique are now being increasingly reported and hailed as advances, and are, for example, evident in Germany where almost two-thirds of the patients with aortic stenosis are now treated by TAVI. Interestingly, the number of SAVR has not decreased. Obviously, the indications there do not always follow the guidelines. In my opinion, the enthusiasm surrounding the procedure has been encouraged and augmented by our colleague cardiologists who see it as yet another step to their independence from the surgeons, and by the young surgical generation, who see in these technologies an opportunity for self-affirmation in a six-decade old specialty, which appeared quite static after the pioneer and heroic early decades of the fifties to the eighties.3
Recently, some authors have raised concern about potential “conflicts of interest among authors and researchers of European clinical guidelines in cardiovascular medicine”. Hinton et al.4 analyzed five of the key European Society of Cardiology clinical practice guidelines, including valvular heart disease and concluded that “the majority of the doctors (80%) that write clinical guidelines have a relevant financial conflict of interest. In addition, industry sponsorship of studies is frequent, and authors are often conflicted with the study funder”. The authors “propose that physicians that write clinical guidelines should be free of such financial conflicts of interest to maintain scientific integrity and independence in the clinical guidelines”. The recently published ACC/AHA guideline for the management of patients with valvular heart disease, also raises many ethical concerns.5
Again, I do not want to be seen as the Old Man of Restelo, who does not believe in progress. In fact, I regularly send patients for TAVI that are referred to me for SAVR. But I have been a personal witness to the unprecedented progress in our cardiac surgery specialty in the last forty-five years, and have also seen the cautious introduction of mechanical and biological prostheses, as well as other medical devices, after long and closely scrutinized laboratory and clinical trials. The more liberal European rules, by contrast with those of the other side of the Atlantic, which now place Europe at the forefront of these developments, may not necessarily be all beneficial.
And I am, especially, a witness to a significant evolution in aortic valve replacement surgical technique, with zero or near-zero tolerance for surgical complications.6 The many positive reports of clinical use of TAVI, some from relatively large series, concluding that the procedure is safe, supported by mortality that is claimed to be ‘similar’ to that of SAVR, some demonstrating good three to five-year durability, may not, and in my view does not, represent the full picture.
In this regard, it is interesting to observe that quite a few years after the introduction of the EuroSCORE II, most published reports on TAVI still use the original EuroSCORE which predicts an average three-fold higher surgical risk. By contrast, recent reports of SAVR in the elderly, demonstrate that current surgical mortality is well below the figures previously reported, now approaching the figures seen in younger patients.
The complications of TAVI are, naturally, also reported in these large series, but it is difficult to ascertain their true incidence, with different rates from series to series. Up to 10% significant periprosthetic leakage (not including regurgitation grade I and II, which would not be tolerated in SAVR), atrio-ventricular block (up to 20%) and migration of the prosthesis are the most frequently reported. The incidence of major vascular complications goes up to 10% of cases, valve malposition in 7%, pericardial effusion and/or tamponade in 12% and aortic rupture in 1%, while leaflet dysfunction was described in seven cases in one series and even two cases of valve septal defect, but less frequent complications, such as coronary obstruction, annular rupture, prosthetic infection, etc. have also been reported. Interestingly, these values are not very dissimilar to those recently reported from our National Registry.7
And here is where one problem arises. The most important scientific journals now struggle with shortage of print space, adopting very selective and restrictive policies on accepting manuscripts for publication. Currently, close to eighty percent of the manuscripts submitted for publication to the mainstream journals are not accepted, despite the good scientific material they may contain. And one of the most affected type of manuscripts are the Case Reports. Currently, only very unique cases are published.
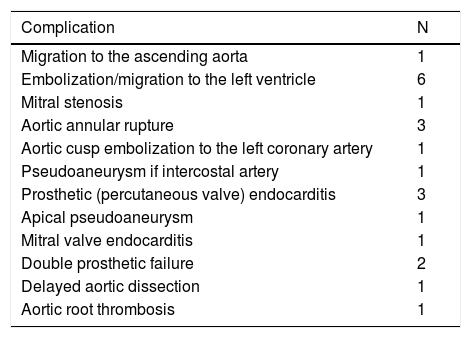
And the number of case reports on complications of TAVI I have reviewed in recent years is fast increasing. This means that our ordinary readers do not have access to important and substantial amount of information. As an active editorial worker responsible for the evaluation and final recommendation to the Chief Editors in this area, I have, for quite some time now, been worried about it and wondered about an ethical way to let it be known to the surgical community. I looked over the list of submissions I reviewed, published and did not publish, in the past four years, and summarized this information in Table 1.
Case reports of complications of transcatheter aortic valve replacement that I reviewed in recent years for several journals, mostly unpublished*.
| Complication | N |
|---|---|
| Migration to the ascending aorta | 1 |
| Embolization/migration to the left ventricle | 6 |
| Mitral stenosis | 1 |
| Aortic annular rupture | 3 |
| Aortic cusp embolization to the left coronary artery | 1 |
| Pseudoaneurysm if intercostal artery | 1 |
| Prosthetic (percutaneous valve) endocarditis | 3 |
| Apical pseudoaneurysm | 1 |
| Mitral valve endocarditis | 1 |
| Double prosthetic failure | 2 |
| Delayed aortic dissection | 1 |
| Aortic root thrombosis | 1 |
* Does not include two papers (published), dedicated specifically to the analysis of complications occurring in two large series of patients.
I emphasize that these cases cannot be interpreted as true indicators of the incidence of these complications. We can only assume that this series of reports is strongly tilted toward the successful stories and that there must be many others which did not have a happy ending and which will never be known unless included in published series. Three things, however, are quite obvious: First, there was an array of unusual and often unexpected complications which mean that TAVI still has a long evolution to follow before it can be compared and compete with the gold standard – SAVR.3 Second, in many circumstances, the patients were consequently underwent, and many saved by, a surgical procedure which was previously considered impossible or too risky. At best, this raises the question of the appropriateness of the initial indication. And thirdly, I continue to be amazed by the immense tolerance demonstrated toward the high incidence of periprosthetic leakage and pacemaker implantation, as if there were no significant clinical, social or economic consequences. There is already evidence of greater late mortality in TAVI in some studies that were early hailed as being very favorable to this method.
Finally, one problem that is seldom mentioned – the cost of the procedures. In countries like ours, TAVI costs roughly twice as much as SAVR, when there are no complications, because the cost of the prosthesis is ten times higher. This promises to be an important burden on our hospitals’ budgets – in Portugal, the number of procedures has quadrupled in the last five years.7 But these values may change considerably. I have just seen a patient who recently had TAVI, in a very experienced center, in whom two prostheses were used because the first one tried could not cross the aortic arch. Actually, use of a second prosthesis is not infrequent, for that and other reasons!
I shall leave to the readers of this Journal to draw their own conclusions.
Note: I have deliberately not mentioned percutaneous mitral and tricuspid valve intervention so as not to confuse the issue, but similar considerations apply.
Conflicts of interestThe author has no conflicts of interest to declare.