Thrombosis is involved in the onset and progression of ST-segment elevation myocardial infarction (STEMI). The aim of this study was to explore the expression level of serum- and glucocorticoid-inducible kinase 1 (SGK1) in intracoronary thrombus and the relationship between them.
MethodsWe identified 30 patients who received treatment in the Affiliated Hospital of Hangzhou Normal University between May 2018 to May 2020 and who underwent thrombus aspiration and percutaneous coronary intervention for ST-segment-elevation myocardial infarction. Additionally, we selected 30 patients with normal coronary angiography for the control group. We analyzed thrombus protein expression profiling by combining tandem mass tag labeling, high-performance liquid chromatography fractionation and mass spectrometry quantification approach.
ResultsThe differentially regulated protein profiles revealed the alteration of serine-threonine/tyrosine-protein kinase, which was upregulated significantly in the experimental group. We selected SGK1 downstream factor for validation and found that the expression of SGK1 in the thrombus of STEMI was significantly increased. Immunohistochemistry (IHC) showed significantly increased expression of SGK1 in the thrombus of STEMI patients compared with the control group (17.21±2.36 vs. 4.14±1.17%, p<0.05). Similar findings were observed in the Western blot analysis (p<0.05). IHC showed that SGK1 expressed in a region similar to that of the platelets.
ConclusionThis is the first quantitative proteomics study to assess thrombus in patients with STEMI. The expression of SGK1 in thrombus was significantly higher in patients with acute STEMI than in the control group. SGK1 might be involved in the platelet physiological process.
A trombose está implicada no início e na progressão do enfarte do miocárdio com elevação do segmento ST (STEMI). O objetivo deste estudo consistiu na avaliação dos níveis de SGK1 no trombo intracoronário e a sua relação entre si.
MétodosIdentificámos 30 doentes tratados no Affiliated Hospital of Hangzhou Normal University entre maio de 2018 e maio de 2020 que se submeteram a aspiração de trombo e a intervenção coronária percutânea por enfarte agudo do miocárdio com elevação do segmento ST. Adicionalmente, selecionámos 30 doentes com angiografia coronária normal para grupo controlo. Analisámos o perfil de expressão de proteínas do trombo.
ResultadosOs perfis proteicos revelaram alterações da serina-treonina/tirosina-proteina cinase que se apresentava sobreregulada no grupo experimental. Selecionámos o fator SGK1 para validação e detetamos que a expressão do SGK1 no trombo do STEMI estava significativamente aumentada. A imuno-histoquímica (IHC) demonstrou o aumento significativo da expressão SGK1 no trombo dos doentes com STEMI comparados com os do grupo controlo (17,21±2,36 versus 4,14±1,17%, p<0,05). Achados semelhantes foram observados na análise western blot (p<0,05). A IHC mostrou que o SGK1 se expressou numa região semelhante à das plaquetas.
ConclusãoEste é o primeiro estudo proteómico quantitativo de avaliação de trombo em doentes com STEMI. A expressão do SGK1 no trombo foi significativamente mais elevada nos doentes com STEMI do que no grupo controlo. O SGK1 pode estar envolvido no processo fisiológico da função plaquetária.
ST-elevation myocardial infarction (STEMI) is one of the most common acute and serious cardiovascular diseases. It is reported that the formation of intracoronary thrombus is one of the important pathogene these for all acute STEMI.1 In 2015 alone, an estimated 15.9 million cases of acute myocardial infarction (AMI) were reported. AMI is the most common manifestation of acute coronary syndrome (ACS), occurring due to coronary thrombosis and a reduction in myocardial perfusion.2
In recent years, AMI has been viewed as an inflammatory disease. So far, aspirin and statins are the only widely used drugs with anti-inflammatory effects in ischemic heart disease.3
Serum- and glucocorticoid-regulated kinase 1 (SGK1) is an enzyme that is encoded by the SGK1 gene in humans. SGK1 belongs to the family of serine/threonine kinases, and its coding region was originally isolated from rat mammary tumor cells.4 SGK1 contributes to the regulation of transport, hormone release, inflammation, cell proliferation and apoptosis. In recent years, there has been increasing evidence that SGK1 expression is regulated during hypertension, diabetes and coronary heart disease.5,6
Arterial thrombosis results from clot formation in the setting of atherosclerotic plaque rupture, leading to platelet aggregation, thrombus formation, vessel occlusion and possible AMI.7 Underlying thrombosis-associated mechanisms may be multidimensional and have not been fully defined. Therefore, insights into the mechanisms of thrombosis may improve our understanding of ACS and promote the development of new therapeutic tools. In this study, we aimed to identify differentially regulated proteins associated with thrombosis in patients with STEMI. We used thrombus aspiration to obtain thrombus components from the coronary arteries of patients undergoing treatment for acute STEMI. By applying the mass spectrometry approach to evaluate signaling pathways associated with thrombosis in STEMI patients, we demonstrate a crucial role for SGK1 expressed in thrombosis and provide a novel mechanistic link between SGK1 and thrombosis.
Materials and methodsStudy designEthical approvals were obtained from the Ethics Research Committee of the Affiliated Hospital of Hangzhou Normal University and informed consent was obtained from all patients. The diagnosis of STEMI was based on the Fourth Universal Definition of Myocardial Infarction.8 Exclusion criteria included the presence of acute and chronic infectious diseases.
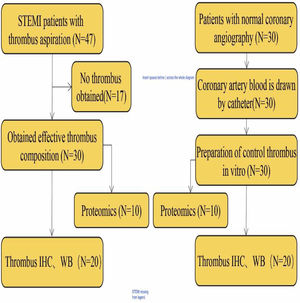
Patients diagnosed with STEMI and high thrombus load in the Affiliated Hospital of Hangzhou Normal University from May 2018 to May 2020 were enrolled in the current study. Of these, 47 patients underwent thrombus aspiration and primary percutaneous coronary intervention (PCI). After further evaluation, 30 patients with effective thrombus composition were selected for subsequent histological examination. An additional 30 patients with normal coronary angiography were included as a control group and their coronary blood was obtained. Figure 1 shows a summary of the study design.
Thrombus aspiration in STEMI patientOral aspirin (300 mg) and ticagrelor (180 mg) were assigned to each patient in the emergency room and heparin (80-100 IU/kg) was given before insertion of coronary guide wire. The Seldinger method was used for the approach in all patients through the right radial artery or femoral artery.9 It was verified by coronary angiography that the infarct-related artery had a high thrombus load, and patients with multi-vessel lesions were only treated for the main culprit vessel. A manual TA catheter (Export AP 6F, Medtronic, Santa Rosa, CA, USA) was used for repeated aspiration for two to three times. Further, standard PCI technique with stent implantation was performed. The above operation was carried out by two specialists with rich intervention experience.
Thrombus burden was classified by the Thrombolysis In Myocardial Infarction (TIMI) thrombus grade (higher thrombus grade, greater thrombus burden)10: Grade 0: no thrombus; Grade 1: possible thrombus; Grade 2: small thrombus, with greatest dimension <1/2 the vessel diameter, Grade 3: moderate thrombus, with greatest dimension >1/2 but <2 vessel diameters; Grade 4: large-sized thrombus with greatest dimension >2 vessel diameters; Grade 5: total vessel occlusion due to thrombus.
Preparation of artificial thrombusFor the control group, we added 1 ml arterial blood and 1 ml tissue factor (peprotech, USA) to cell culture medium (RPMI-1240+10% fetal calf serum) and incubated at 37°C for six hours to prepare the control thrombus.
Management of obtained thrombusThe obtained thrombus tissue was processed immediately: one part of thrombus was stored in a liquid nitrogen container for the proteomics analysis and western blot analysis. The other part was fixed in 4% paraformaldehyde for 24 hours and processed routinely for paraffin-embedding and immunohistochemistry (IMC) analysis.
Proteomics analysisTotal protein was extracted from the control and pathological thrombus and underwent reduction followed by alkylation. Trypsin-digested peptides were separated by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. The resulting MS/MS data were processed using the Maxquant software (v.1.5.2.8). For detailed procedures, see the supplementary material.
We quantified approximately 2228 proteins and 17 653 unique peptides were quantified in reference to the database of SwissProt. To discriminate the differentially expressed proteins, a two-tailed t-test was applied to the two groups: thrombus-STEMI (disease) and thrombus (control). The coefficient variation (CV) of each protein in the two groups was calculated. At p<0.05 (or CV<0.1 or none), the differential expression level changes >1.3 was considered as the significant up-regulation change threshold and <1/1.3 as the significant downregulation change threshold.
Validation of proteinTo validate the expression of SGK1, we used Western blot analysis and IHC.
Reagents and antibodiesAntibodies against SGK1 (ab59337), CD68 (ab213363), Fibrinogen (ab92572), Glycophorin A (ab129024) and CD31 (ab28364) were purchased from Abcam (Cambridge, UK); and a horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG antibody (A0208) was purchased from Beyotime (Shanghai, China).
ImmunohistochemistryBriefly, 3 mm thick sections mounted on glass slides were processed for IHC. All paraffin sections were dewaxed in xylene and dehydrated in an alcohol gradient. Antigen was repaired by high-pressure antigen repair with sodium citrate buffer for 10 m. Then endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 10 m (in dark). About 5% of bovine serum albumin was used to block nonspecific binding by incubating sections for 30 min at room temperature, followed by incubation with SGK1 antibodies at 4°C overnight. Sections were then incubated with a secondary antibody at room temperature for 20 m. Diaminobenzidine was used as a chromogen resulting in brown staining of positive cells (after washing three times with phosphate-buffered saline between each step). The nuclei were counterstained using hematoxylin then observed under a microscope and imaging used to assess tissue staining. Brown particles present in the cytoplasm and/or nuclei were considered positive signals.
Western blot analysisThrombus tissues were shred using shears then RIPA buffer and PMSF were added in the proportion of 1 ml RIPA+10 μl PMSF+100mg tissue at 4°C for 30 min (Solarbio, Beijing, China). Lysates were centrifuged at 12 000 g for 30 min at 4°C. Protein concentrations of the supernatants were determined using the BCA protein assay reagent kit (Beyotime). Then, the supernatants containing total proteins were mixed with SDS loading buffer and heated at 95°C for 10 min. About 40 mg of total protein from each sample was concentrated on 10% Tris-glycine SDS gels, separated on 12% Tris-glycine SDS gels and transferred to polyvinylidene fluoride membranes. Membranes were blocked with 5% non-fat dry milk in tris-buffered saline (TBST) for 1 h and incubated overnight with the SGK1 antibody at 4°C. After washing three times for 15 min with TBST (in a shaker), membranes were incubated with HRP-conjugated secondary antibodies at 37°C for 1 h. The bound antibodies were visualized using the chemiluminescence gel imaging system (Microchemi 4.2, Israel).
Statistical analysisAll statistical analyses were performed using SPSS 23.0 (IBM Inc., Chicago, Illinois, USA). The values were shown as means ± standard deviation (SD). Principal component analysis, relative standard deviation and Pearson's correlation coefficient were used to evaluate protein quantitative repeatability. Significant differences were calculated using the Newman-Keuls test and two-tailed Student's t-test. Categorical variables were reported as numbers and percentages and comparisons between two groups were performed using Chi-square or Fisher's exact test when the expected cell value was less than five. Continuous variables were presented as mean ± standard deviation and Student's t-test was applied for normally distributed variables. Non-normally distributed data were presented as a median (first quartile, third quartile) and compared with the Mann-Whitney U-test. A two-tailed p<0.05 was considered statistically significant.
ResultsBaseline characteristics of patientsA total of 60 patients ranging from 31-89 years old were enrolled in this study. The STEMI group consisted of 30 patients with effective thrombus composition while the control group consisted of 30 patients with normal coronary angiography. As shown in Table 1, demographic variables including age, sex and body mass index were well balanced between groups. However, there were significant differences in the risk factors between the two groups, which were consistent with the European Society of Cardiology guidelines.9 Blood test before the intervention showed significant increase in the serum level of cTNI (50.0 vs. 0.02 ng/ml, p<0.05), creatinine kinase MB fraction (109.9 vs. 11.5 U/L, p<0.05), total cholesterol (4.5 vs. 3.9 mmol/L, p<0.05), triglyceride (1.5 vs. 1.2 mmol/L, p<0.05), high sensitivity C-reactive protein (8.6 vs. 2.0 mg/L, p<0.05), blood sugar (7.2 vs. 5.5 mmol/L, p<0.05) and creatinine kinase (1494.6 vs. 69.0 U/L, p<0.05) in the STEMI group. Echocardiography before intervention showed a significant increase in left ventricular ejection fraction (53.0 vs. 60.7%, p<0.05).
Baseline characteristics of all included patients.
| STEMI Group (N=30) | Control Group (N=30) | p value | |
|---|---|---|---|
| Demographics | |||
| Age (mean±SD), years | 56.1±14.6 | 57.4±12.6 | 0.721 |
| Male, n (%) | 25(83.3) | 23(76.7) | 0.748 |
| BMI (mean±SD), kg/m2 | 25.9±4.6 | 24.2±3.4 | 0.132 |
| Risk factors | |||
| Hypertension, n (%) | 13 (43.3) | 16 (53.3) | 0.606 |
| Diabetes mellitus, n (%) | 14(46.7) | 3 (10.0) | 0.002* |
| Dyslipidemia, n (%) | 6 (20.0) | 1 (3.3) | 0.103 |
| Hyperuricemia, n (%) | 9(30.0) | 4(13.3) | 0.209 |
| Smoker, n (%) | 20 (66.7) | 6 (20.0) | 0.001* |
| Drinker, n (%) | 9(30.0) | 8(26.7) | 1.000 |
| Prior MI, n (%) | 4 (13.3) | 0 (0) | - |
| Prior PCI, n (%) | 5 (16.7) | 0 (0) | - |
| Prior CABG, n (%) | 0 (0) | 0 (0) | - |
| Laboratory findings | |||
| cTNI (x±s, ng/ml) | 50.0 (21.6, 116.5) | 0.02(0.02, 0.04) | 0.000* |
| CK (x±s, U/L) | 1494.6 (646.7, 3414.9) | 69.0 (59.0, 89.5) | 0.000* |
| CK-MB (x±s, U/L) | 109.9 (47.9, 248.0) | 11.5 (8.0, 13.3) | 0.000* |
| TC (x±s, mmol/L) | 4.5±1.0 | 3.9±0.7 | 0.020* |
| TG (x±s, mmol/L) | 1.5(1.1, 2.0) | 1.2 (0.9, 1.5) | 0.040* |
| LDL-C (x±s, mmol/L) | 2.4±0.8 | 2.2±0.6 | 0.289 |
| HDL-C (x±s, mmol/L) | 1.0±0.2 | 1.2±0.3 | 0.000* |
| hs-CRP (x±s, mg/L) | 8.6 (3.8, 18.4) | 2.0 (0.8, 3.5) | 0.000* |
| LVEF (%) | 53.0±9.9 | 60.7±7.3 | 0.002* |
| Blood Sugar (x±s, mmol/L) | 7.2±2.1 | 5.5±1.1 | 0.001* |
Normally distributed continuous data are presented as mean ± standard deviation and non-normally distributed data as a median (first quartile, third quartile). Categorical data are presented as frequencies (percentages).
p<0.05.
BMI: body mass index; CABG: coronary artery bypass graft; CK: creatinine kinase; CK-MB: creatinine kinase MB; cTNI: cardiac troponin I; HDL-C: high-density lipoprotein; hs-CRP: high sensitivity C-reactive protein; LDL-C: low-density lipoprotein; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; TC: total cholesterol; TG: triglyceride.
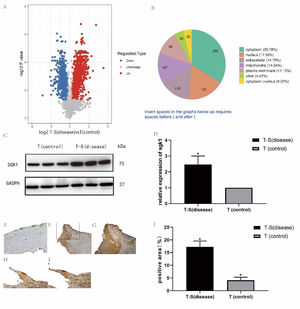
The log2 ratios (fold change) of disease versus control were plotted against the -log10 (p values), resulting in the volcano plot (Figure 2). Multiscatter plot of peptide intensities resulted in the Pearson correlation coefficients higher than 0.90, attesting to the high grade of reproducibility of biological replicates. Approximately 746 and 450 proteins were upregulated and downregulated, respectively, with a fold change higher than 1.3 in the thrombus-STEMI compared with the control thrombus.
(A) Distribution of differentially regulated proteins in thrombus-STEMI. The volcano plot obtained by the two-sided t-test of the two groups: thrombus-STEMI (disease) vs thrombus (control). Dots on the left indicates the downregulated proteins while dots on the right represents the upregulated proteins. (B) Upregulated proteins are mainly derived from the cytoplasm.
The majority of upregulated proteins in the thrombus-STEMI originated from the cytoplasmic nucleus (Figure 2). SGK1 protein was upregulated with a ratio of 1.926. Similarly, the proto-oncogene tyrosine-protein kinase Src(SRC) was also upregulated in the intracoronary thrombosis with a ratio of 2.635(Table 2). The expression of SGK1 was further validated used Western blot analysis and IHC.
Differentially regulated proteins.
The peak intensity area of each protein band was calculated using ImageJ software. As shown in Figure 3, the relative abundance of SGK1 protein in the thrombus was analogous to the results from the label-free quantification study.
(A-B) Validation of SGK1 expression using Western blot analysis. The results are expressed as mean values ± SEM. The x-axis represents the thrombus-STEMI and control groups and the y-axis represents the relative expression of the respective protein to the control. Differences were considered significant when *p<0.05. GADPH was used as a housekeeping protein. (C) Representative images from the IHC analysis for SGK1 in the thrombus-control group. (D-E) Representative images from the IHC analysis for SGK1 in the thrombus-STEMI group. (F-G) High expression of SGK1 in the thrombus platelets: (F) Platelet (SGK1 antibody); (G) platelet (CD31 antibody). (H) The results are expressed as mean values ± SEM. The x-axis represents the thrombus-STEMI and control groups and the y-axis represents the percentage of SGK1 positive area per of thrombus. Original magnification, ×200 (C and D) or ×400 (E, F, and G). Differences were considered significant when *p<0.05.
Imaging analysis in IHC was performed using ImageJ software. By comparing the percentage of SGK1 positive area between the thrombus-STEMI and control groups, we found that the expression of SGK1 in the thrombus of STEMI was significantly increased (Figure 3). The IHC results were also consistent with the label-free quantification study. SGK1 was expressed in the macrophages and platelets, but mainly in platelets, which were mainly distributed around the thrombus (Figure 3).
DiscussionThrombosis is involved in the onset and progression of STEMI.9 A comprehensive exploration of the molecular mechanisms of STEMI is crucial in the discovery of new therapeutic strategies for the treatment of STEMI. Mass spectrometry-based proteomics has been widely applied to identify new biomarkers and therapeutic targets.11 In the present study, for the first time, we describe the change in the proteomic profile of thrombus in patients with STEMI.
Patients have a high risk of subsequent ischemic events following ACS, each episode is associated with an increase in mortality. Heightened predisposition to atherothrombotic events may persist for years.12 Therefore, treatment by effectively preventing the rupture of coronary artery plaque and thrombosis is worth exploring. The concept that inflammation participates pivotally in the pathogenesis of atherosclerosis and its complications has gained considerable attention but is yet to enter clinical practice.13 SRC is involved in increasing the phosphorylation activity of various non-receptor tyrosine kinases such as CSK (c-src tyrosine kinase).14 The CSK protein involved in cell growth, adhesion and polarization which were also upregulated in intracoronary thrombosis.15 Based on our results, serine/threonine kinases might play an important role in the pathogenesis of acute STEMI along with these proteins (Table 2). SGK1 is a factor downstream of phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway.16 It is believed that excessive SGK1 expression and activity, contribute to the pathophysiology of several disorders, including hypertension, obesity, diabetes, thrombosis, stroke, fibrosing disease, infertility and tumor growth.17 However, the correlation between SGK1 and thrombosis is yet to be confirmed by large, double-blind, randomized controlled studies. In this study, SGK1 proteins were highly upregulated. The increased expression of SGK1 may enhance thrombus formation in the coronary artery.
Our results revealed the important role of SGK1 in coronary thrombosis by using thrombus aspiration in STEMI patients. We found that the expression of SGK1 protein was increased in the thrombus of STEMI patients. In addition, we found that the SGK1 protein played an important role in the platelet physiological process during the coronary thrombosis process. Additionally, the SGK1 protein was expressed particularly in the platelets, which were mainly distributed around the thrombus.
In the baseline characteristics of the population, there was significant difference in the prevalence of diabetes and smoking rate among two groups. Compelling evidence points to a role of SGK1 in the development of diabetes and the pathophysiology of its complications, which may affect the expression of SGK1 in serum level of STEMI patients.18 However, previous studies have indicated that SGK1 is mainly expressed in the kidneys, pancreas and adipose tissue of diabetics, And there is no evidence that SGK1 is highly expressed in the platelets of diabetics.19–21 Risk factors for cardiovascular disease are well-established in diabetes. Sheng's study22 provides evidence linking diabetes status with vulnerable features of coronary plaques in patients with STEMI. Meanwhile, the SGK1 inhibitor EMD638683 blocking NLRP3 inflammasome activation, and the NLRP3 is of immense interest currently in treating atherothrombosis.23,24 It is reasonable to believe that SGK1 plays an important role in the process of thrombosis. Additionally, although smoking is a risk factor for cardiovascular disease, there is no evidence of SGK1 being associated with it currently.
Numerous previous studies have established the importance of PI3K-dependent signaling responses of monocytes and macrophages to atherogenic mediators promoting atherosclerosis.25 SGK1 is the major downstream effector of PI3K-dependent signaling. Borst et al.,26 have shown that SGK1 plays a central role in vascular inflammation during atherogenesis. They also found that SGK1 is involved in the regulation of monocyte/macrophage migration and matrix metalloproteinase 9 (MMP-9) transcription via regulation of nuclear factor-kappa B (NF-κB). It follows that the relationship between SGK1 and arteriosclerosis is inseparable.
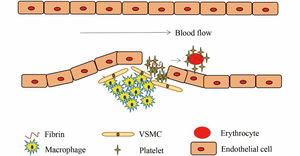
Structurally, arterial and venous thrombi are distinct. Arterial thrombosis occurs under high shear flow when platelet-rich thrombi are formed around ruptured atherosclerotic plaques and damaged endothelium (Figure 4).7 When the vascular endothelium is damaged, the subendothelial extracellular matrix (ECM) is exposed. The platelets adhere to the ECM and then they activate, swell and deform, releasing the related media, which promotes the activation, adhesion and aggregation of platelets, thus forming the platelet heap. This process is repeated and the small platelet pile increases gradually, eventually leading to a platelet thrombus.
Earlier studies have shown that the activation of platelets by thrombin leads to the upregulation of SGK1 protein, which is expected to further enhance activation-dependent Ca2+ entry into the platelets with a subsequent Ca2+-dependent degranulation, adhesion, aggregation and thrombus formation.16,27 Other studies have shown that the PI3K pathway downstream SGK1 effector is a novel regulator of platelet granule biogenesis.28 Since platelet granules are essential in thrombosis, therefore, regulating the mechanism of platelet adhesion is of great significance for acute myocardial infarction. SGK1 upregulates the transcription level of RAB27B, which was identified as a major player in the dense granule biogenesis and secretion.28 Similarly, platelet migration could be stimulated by stromal cell-derived factor-1 (SDF-1), an effect dependent on PI3K.29
In this study, we found that SGK1 was expressed mainly in platelets. The expression of SGK1 was significantly higher in the thrombus-STEMI than the control group. We hypothesized that SGK1 could promote the invasion of platelets into the inflamed blood vessel wall and accelerate the development of arteriosclerosis plaque. When the plaque ruptures, SGK1 could accelerate the aggregation and chemotaxis of platelets and promote thrombosis. In light of these observations, it is tempting to speculate that enhancing the expression of SGK1 might contribute to inflammation within atherosclerotic plaques and subsequent progression of atherogenesis.
In summary, our results showed that the expression of SGK1 in thrombus was significantly higher in patients with acute STEMI. SGK1 might be involved in the platelet physiological process, but the mechanism of action of SGK1 warrants further validation. SGK1 may present a potential therapeutic target in the prevention of the development of atherothrombotic events.
Study limitationsFirst, this study is a single-center, small-sample size and single-blind nonrandomized trial that did not include patients with unaspirated or few thrombus, which led to selection bias. Moreover, treatment biases might have occurred, as the physicians administering treatment were aware of the study group assignments. Secondly, thrombus aspiration can only obtain most of the thrombus from the coronary artery. However, complete removal of thrombus from the coronary artery may not be achieved, resulting in sample deviation. Thirdly, this study used semi-quantitative methods to analyze the expression of SGK1 in the thrombus, thus leading to information bias. Finally, considering accumulating evidence indicating the SGK1 is highly expressed in diabetic patients, the increased prevalence of diabetes in the STEMI group may have led to biased research results.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by grants from the Zhejiang Provincial Natural Science Foundation (LQ18H020006), Hangzhou Health and Family Planning Technology Plan key projects (2017ZD02), the Planned Science and Technology Project of Zhejiang Province, China (2020KY216), Hangzhou City Health Sciences and Technology Program (OO20190126).