This study aimed to evaluate the performance of non-vitamin K antagonist oral anticoagulation (NOAC) in patients with previous stroke and non-valvular atrial fibrillation (AF) compared with left atrial appendage occlusion (LAAO) in primary and secondary stroke prevention settings.
MethodsThis was a prospective, single-center, non-randomized cohort study of 302 consecutive patients with non-valvular AF and at high risk for stroke. Two treatment strategies were compared: LAAO (n=91) and long-term treatment with NOAC (n=149). The primary outcome was the composite endpoint of death, stroke and major bleeding. Propensity score and cause-of-death analyses were performed to compare outcomes.
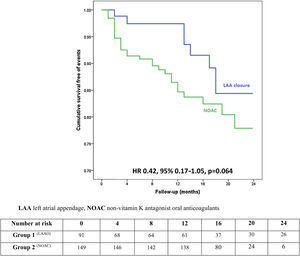
ResultsIn a mean follow-up of 13 months, there were 30 deaths (LAAO 8.8% vs. NOAC 14.8%), five strokes (LAAO 1.1% vs. NOAC 2.7%) and six major bleeds (LAAO 1.1% vs. NOAC 3.4%). There was a non-significant trend for a lower incidence of the primary endpoint in the LAAO group (11.0% vs. 20.9%; HR 0.42, 95% CI 0.17-1.05, p=0.064). Considering only secondary prevention LAAO patients (34.1% of the LAAO group), there was also a non-significant lower incidence of the primary endpoint (LAAO 6.5% vs. 20.9%; HR 0.30, 95% CI 0.07-1.39, p=0.12). While about a fifth of LAAO patients stopped antiplatelet treatment six months after device implantation due to recurrent minor bleeding, no adverse cardiovascular event or major bleeding occurred in this subset of patients.
ConclusionIn this registry-based study, LAAO was a reasonable alternative to NOAC for the prevention of a composite endpoint of all-cause mortality, stroke and major bleeding in patients at high risk for stroke.
Este estudo pretende comparar o desempenho dos anticoagulantes orais não antagonistas da vitamina K (NOAC) em doentes com fibrilhação auricular (FA) não valvular e antecedente de acidente cerebrovascular (AVC) isquémico, em comparação com o encerramento de apêndice auricular esquerdo (AAE) de doentes em prevenção primária e secundária de AVC.
MétodosEstudo unicêntrico, prospetivo, não randomizado, de coorte, constituído por uma amostra de 302 doentes com FA não valvular e elevado risco de AVC. Foram comparadas duas estratégias terapêutica: encerramento de AAE (n=91) versus tratamento com NOAC (n=149), através do modelo de propensity score. O desfecho primário era composto por morte, AVC e hemorragia major.
ResultadosO seguimento clínico médio foi de 13 meses, tendo ocorrido 30 mortes (AAE 8,8% versus NOAC 14,8%), 5 AVC (AAE 1,1% versus NOAC 2,7%) e seis hemorragias graves (AAE 1,1% versus NOAC 3,4%). A incidência do desfecho primário mostrou uma redução não significativa no grupo do dispositivo (11,0% versus 20,9%; HR 0,42, 95% CI 0,17-1,05, p=0,064). Considerando apenas os doentes em prevenção secundária de AVC (34,1% do grupo de AAE), a incidência do desfecho primário mostrou uma redução não significativa (AAE 6,5% versus 20,9%; HR 0,30, 95% CI 0,07–1,39, p=0,12). Nos cerca de 20% dos doentes submetidos ao encerramento do AAE que suspenderam a terapêutica antiplaquetária após seis meses da implantação do dispositivo por hemorragias minor, não houve eventos cardiovasculares ou hemorragias graves.
ConclusãoNeste estudo, o encerramento de AAE não foi inferior aos NOAC para prevenção do desfecho primário composto por morte, AVC e hemorragia major.
Left atrial appendage occlusion (LAAO) is considered a reasonable alternative to oral anticoagulation (OAC) for stroke prevention in patients with non-valvular atrial fibrillation (AF) in whom OAC is contraindicated.1 Aging populations and improvements in life expectancy increase the risk of AF and AF-related stroke, which tends to be more incapacitating and more often fatal than other etiologies of stroke.2 Although OAC is the mainstay of cardioembolic stroke prevention, it frequently threatens the delicate balance between stroke prevention and life-threatening bleeding in frail patients.1,2
Evidence supporting LAAO derives mainly from two randomized trials, PREVAIL and PROTECT AF,3 with PROTECT-AF showing the superiority of occlusion in mortality and stroke reduction compared to optimal medical treatment with warfarin in the long term. However, no randomized clinical trials have ever compared device therapy with non-vitamin K oral anticoagulation (NOAC), which is easier to use and appears to provide at least similar stroke reduction as warfarin and to be associated with slightly lower rates of major bleeding.4 Currently, LAAO is not a recommended alternative to long-term NOAC therapy in low bleeding risk patients who are otherwise good candidates for the latter. Furthermore, most data on LAAO5,6 derive from non-randomized assessments of the efficacy of the device based on intrinsic thromboembolic (CHA2DS2-VASc) and bleeding (HAS-BLED) risk, and compared to vitamin K antagonists (VKA). Therefore, it is unclear whether LAAO could be considered a reasonable alternative to NOAC in AF patients at high stroke and bleeding risk.7
This study aimed to evaluate the performance of NOAC in patients with previous stroke and non-valvular AF compared with LAAO in primary and secondary stroke prevention settings.
MethodsStudy designThis single-center, non-randomized cohort study aimed to assess different antithrombotic treatment strategies for cardioembolic stroke prevention. Data were obtained from two registries: the LAAO Registry, comprising consecutive LAA percutaneous closure cases, in primary or secondary stroke prevention (details on LAAO indication, technique and outcomes have been published5); and the Neurology AF registry, comprising consecutive AF-related stroke patients (secondary stroke prevention) admitted to our main tertiary hospital center between 2015 and 2017. The overall purpose of this study was to assess the outcomes of patients on two treatment strategies: LAAO plus antiplatelet therapy (group 1) and long-term therapy with NOAC (group 2).
All patients were prospectively followed at six-month intervals after hospital discharge from the LAAO procedure or AF-related stroke, and follow-up data were last updated in January 2018.
The outcomes of the two groups were compared using cause-of-death, proportional hazards regression and propensity-score (PS) analyses.8
Data collection and analysis were approved by the individual sites’ institutional review boards, and the requirement for written informed consent was waived for each patient. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Study population and characteristicsPatients were included if they were ≥18 years of age, had documented AF and were considered at high risk of stroke, as defined by a CHA2DS2-VASc score of ≥2 or a history of stroke. In addition, patients in the LAAO group were at high bleeding risk, as defined by a HAS-BLED score ≥3 or had a contraindication to OAC.
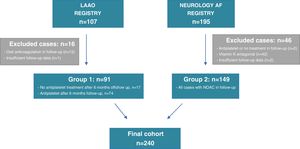
A total of 107 patients were included in the LAAO Registry, comprising those who underwent LAAO, and 195 patients were included in the Neurology AF Registry, comprising AF-related stroke cases that were put on NOAC after the cerebral ischemic event. Of the initially screened LAAO cases, 15 patients were excluded from the analysis as they were prescribed OAC at some point during follow-up. Concerning the Neurology AF Registry, 44 cases were excluded from the initially screened population as they were eventually changed to a VKA or antiplatelet therapy or their antithrombotic treatment was discontinued. Moreover, an additional three patients were excluded due to lack of follow-up data (Figure 1). The final study group comprised 240 patients: 91 cases in group 1 who underwent LAAO, and 149 patients on uninterrupted NOAC after AF-related stroke (group 2).
In the LAAO group, after device-related leak or thrombus were excluded, 18.9% (n=17) discontinued all antithrombotic therapy after the mandatory six-month antiplatelet treatment protocol (one month of dual antiplatelet therapy, followed by at least five months of single antiplatelet therapy) due to recurring minor bleeds (skin, mouth, nose, or eye) and/or patient decision.5 The other patients from group 1 continued single antiplatelet therapy with either aspirin or clopidogrel throughout follow-up.
The data collected included demographics, type of AF (paroxysmal vs. persistent), history of diabetes, hypertension, congestive heart failure, previous cerebrovascular events, CHA2DS2-VASc and HAS-BLED score variables,9,10 renal dysfunction estimated using the Modification of Diet in Renal Disease (MDRD) equation and glomerular filtration rate stratified into different stages (≥60, 30-59, and <30 ml/min), and follow-up antithrombotic drugs.
Study endpointsThe primary study outcome was a composite endpoint of all-cause mortality, non-fatal stroke and major bleeding. The secondary endpoints were a composite of cardiovascular death and non-fatal stroke, to specifically compare the cardiovascular protective effect of each treatment strategy, and major bleeding (safety endpoint).
Vital status data were obtained by the investigators through analysis of death certificates and necropsy results, clinical notes from hospital admissions, and information provided by the patients’ general practitioners when needed. In cases where insufficient information was available to make a reasonable assumption of the cause of death, this was classified as unknown. Stroke was defined as the sudden onset of a focal neurologic deficit in a location consistent with the territory of a major cerebral artery and categorized as ischemic, confirmed by brain computed tomography or magnetic resonance imaging. Transient ischemic attacks without brain imaging demonstrating a new-onset vascular lesion were not considered in this study. Hemorrhagic transformation of an ischemic stroke was still categorized as a stroke event rather than as major bleeding. The safety outcome was major bleeding, which was defined, according to the International Society on Thrombosis and Haemostasis criteria,11 as clinically overt bleeding accompanied by a decrease in hemoglobin level of at least 2 g/dl or transfusion of at least two units of packed red cells, occurring at a critical site, or resulting in death. All other bleeding episodes were considered minor and excluded from the study analysis. Table 1 shows the adjudication of bleedings according to the Bleeding Academic Research Consortium criteria.12
Adjudication of bleeding events according to the Bleeding Academic Research Consortium criteria.12
| Bleeding | Number of events | ||
|---|---|---|---|
| Group 1 (LAAO) | Group 2 (NOAC) | ||
| Type 0 | No bleeding | ND | ND |
| Type 1 | Bleeding that is not actionable and does not cause the patient to seek unscheduled performance of studies, hospitalization, or treatment by a healthcare professional; may include episodes leading to self-discontinuation of medical therapy by the patient without consulting a healthcare professional | ND | ND |
| Type 2 | Any overt, actionable sign of that does not fit the criteria for type 3, 4, or 5 but does meet at least one of the following criteria: (1) requiring nonsurgical, medical intervention by a healthcare professional, (2) leading to hospitalization or increased level of care, or (3) prompting evaluation | ND | ND |
| Type 3a | Overt bleeding plus hemoglobin drop of 3 to <5 g/dl (provided hemoglobin drop is related to bleed); any transfusion with overt bleeding | 0 | 4 |
| Type 3b | Overt bleeding plus hemoglobin drop ≥5 g/dl (provided hemoglobin drop is related to bleed); cardiac tamponade; bleeding requiring surgical intervention for control; bleeding requiring intravenous vasoactive agents | 0 | 0 |
| Type 3c | Intracranial hemorrhage (does not include microbleeds or hemorrhagic transformation); intraocular bleed compromising vision | 0 | 0 |
| Type 4 | CABG-related bleeding | 0 | 0 |
| Type 5a | Probable fatal bleeding; no autopsy or imaging confirmation but clinically suspicious | 1 | 1 |
| Type 5b | Definite fatal bleeding; overt bleeding or autopsy or imaging confirmation | 0 | 0 |
| Total=1 | Total=5 | ||
CABG: coronary artery bypass grafting; LAAO: left atrial appendage occlusion; ND: no data; NOAC: non-vitamin K oral anticoagulation.
Statistical analysis was performed using IBM SPSS Statistics, version 24 (IBM, Armonk, New York). Baseline characteristics were reported as mean ± standard deviation for continuous data and as counts and proportions for categorical data. The Kolmogorov–Smirnov test was used to test the normal distribution of continuous variables. The chi-square test, Student's t test, and equivalent non-parametric tests were used when appropriate. Two-sided p-values <0.05 were considered to indicate statistical significance.
Four different PS methods have been proposed to remove the effects of confounding when estimating the effects of treatment on outcomes: propensity score matching, stratification on the propensity score, inverse probability of treatment weighting using the propensity score, and covariate adjustment using the propensity score.8 In the latter approach, the outcome variable is regressed on an indicator variable denoting treatment status (LAAO or oral anticoagulation in this case) and the estimated propensity score, in addition to any other variables considered relevant to outcome prediction. To obtain the PS, all baseline covariates were included that were shown to affect the outcome, and other parameters that were previously seen to be of prognostic importance in these populations7: age, type of AF (paroxysmal vs. persistent/permanent), CHA2DS2-VASc score and HAS-BLED score.
For the primary (composite) outcome, patients were censored when the first event occurred and groups were compared using proportional hazards regression with adjustment for the PS and all mortality predictors. For the secondary endpoints, competing risk regression was performed to obtain subdistribution hazard ratios (HR). This was carried out to account for the competing risks – non-cardiovascular death when assessing the composite endpoint of cardiovascular death and stroke, and all-cause death when assessing the occurrence of major bleeding. For associations, we used a two-sided test of significance at the p<0.05 level.
A subgroup analysis of secondary prevention patients was also performed, including group 1 individuals with a history of stroke plus all group 2 patients.
ResultsThe baseline characteristics of the overall population are reported in Table 2. LAAO patients were younger, more often male, and had a higher prevalence of paroxysmal AF and history of congestive heart failure, and lower CHA2DS2-VASc (4.3 vs. 5.3; p<0.001) and HAS-BLED scores (3.0 vs. 4.0; p<0.001) than those on NOAC (group 2). Regarding major LAAO procedural complications, no pericardial tamponade, stroke or death was observed.
Baseline characteristics of the study population (n=240).
| Group 1 (n=91) | Group 2 (n=149) | p | |
|---|---|---|---|
| Age, years | 74.7±8.7 | 77.8±8.0 | <0.007 |
| Male, % | 64.8 (59) | 46.3 (69) | 0.005 |
| AF, % | 0.012 | ||
| Paroxysmal | 26.4 (24) | 13.4 (20) | |
| Persistent/permanent | 73.6 (67) | 86.6 (129) | |
| Hypertension, % | 86.8 (79) | 82.6 (123) | 0.38 |
| Diabetes, % | 35.3 (32) | 24.2 (36) | 0.07 |
| Dyslipidemia, % | 49.5 (45) | 54.4 (81) | 0.46 |
| Previous stroke/TIA before implantation or index event, % | 34.1 (31) | 30.9 (46) | 0.61 |
| Congestive heart failure, % | 46.2 (42) | 22.8 (34) | <0.001 |
| Vascular disease, % | |||
| Coronary artery disease | 14.3 (13) | 14.8 (22) | 0.91 |
| Carotid artery disease | 2.2 (2) | 31.5 (47) | <0.001 |
| Peripheral arterial disease | 3.3 (3) | 1.3 (2) | 0.30 |
| Mean CHA2DS2-VASc score | 4.3±1.4 | 5.3±1.3 | <0.001 |
| Mean HAS-BLED score | 3.0±0.9 | 4.0±0.7 | <0.001 |
| Renal dysfunction, % | 0.59 | ||
| GFR ≥60 ml/min | 72.7 (66) | 67.5 (101) | |
| GFR 30-59 ml/min | 21.2 (19) | 29.8 (44) | |
| GFR <30 ml/min | 6.1 (6) | 2.6 (4) | |
| Mean follow-up in surviving patients, months | 13.7±8.5 | 12.8±6.2 | 0.19 |
| Follow-up >12 months, % | 50.6 (46) | 51.7 (77) | |
AF: atrial fibrillation; GFR: glomerular filtration rate, Group 1: left atrial appendage occlusion plus antiplatelet therapy or no antithrombotic drugs; Group 2: non-vitamin K antagonist oral anticoagulation; TIA: transient ischemic attack.
During a mean follow-up of 13±7.2 months (interquartile range 7.3-18.0 months) after hospital discharge, 30 patients died: eight following LAAO (8.0% mortality/year) and 22 after hospital discharge from the index stroke (13.4% mortality/year). There were no hemorrhagic strokes and no vascular or coronary ischemic events were reported throughout follow-up. Table 3 lists all causes of death, as well as the number of patients experiencing any of the secondary endpoints, and shows that all ischemic (n=4, mean time to stroke: five months) and hemorrhagic events (n=5, mean time to bleeding: four months) in NOAC patients occurred in the first year of follow-up. By contrast, in the LAAO group, the two major events reported, stroke and major bleeding, occurred after the first 12 months of follow-up (23 and 14 months, respectively). Although mortality rates were similar between the study groups, all-cause mortality occurred more frequently in the first year of follow-up in the NOAC group than in those receiving LAAO (25.0% of all deaths in the latter group vs. 77.3% in the former).
Mortality, vascular and bleeding events (n=240).
| Group 1 (n=91) | Group 2 (n=149) | p | |
|---|---|---|---|
| All-cause mortality, % | 8.8 (8) | 14.8 (22) | 0.18 |
| Causes | |||
| Cancer | 3 | – | |
| Respiratory disease | 0 | 9 | |
| GI/urinary disease | 1 | 1 | |
| Bleeding diathesis | 1 | 1 | |
| CV/cerebrovascular | 0 | 2 | |
| Unknown | 3 | 9 | |
| CV mortality, % | 0 | 1.3 (2) | 0.85 |
| Stroke, % | (1) | 2.7 (4) | 0.40 |
| Major bleeding, % | 1.1 (1) | 3.4 (5) | 0.28 |
| 0-12 months of follow-up | n=45 | n=72 | |
| All-cause mortality, % | 4.4 (2) | 23.6 (17) | 0.010 |
| CV mortality, % | 0 | 3.0 (2) | 0.26 |
| Stroke, % | 0 | 5.6 (4) | 0.12 |
| Major bleeding, % | 0 | 6.9 (5) | 0.82 |
| 13-24 months of follow-up | n=46 | n=77 | |
| All-cause mortality, % | 13.0 (6) | 6.5 (5) | 0.27 |
| CV mortality, % | 0 | 0 | – |
| Stroke, % | 2.2 (1) | 0 | 0.21 |
| Major bleeding, % | 2.2 (1) | 0 | 0.21 |
CV: cardiovascular; GI: gastrointestinal; Group 1: left atrial appendage occlusion plus antiplatelet therapy or no antithrombotic drugs; Group 2: non-vitamin K antagonist oral anticoagulation;
Crude incidence rates of the primary endpoint were 86.6 and 192.1 events per 1000 patient-years in those receiving LAAO and NOAC, respectively. The secondary outcome of cardiovascular death and stroke occurred at a rate of 9.6 and 37.3 events per 1000 patient-years in LAAO and NOAC patients, respectively. Crude major bleeding incidences were 9.4 and 31.1 events per 1000 patient-years in the LAAO and NOAC groups, respectively.
In univariate analysis, age (p=0.049), type of AF (p=0.017), history of carotid artery disease (p=0.038) and HAS-BLED score (p=0.025) predicted the primary endpoint.
Primary and secondary outcomes with propensity score adjustmentThere was a trend for a lower risk of the primary endpoint in the LAAO group on Cox regression analysis with adjustment for the PS and all predictors of the endpoint (HR 0.42, 95% confidence interval [CI] 0.17-1.05, p=0.064), as depicted in Figure 2. The effect size was particularly large among female patients (HR 0.12, 95% CI 0.01-1.25, p=0.076). On average, there was one additional primary outcome for every 11 patients receiving NOAC compared with the same number of LAAO patients (Table 4).
Adjusted Cox regression analysis for the primary endpoint of death, stroke and major bleeding.
| HR | 95% CI | p | |
|---|---|---|---|
| Age | 1.027 | 0.976-1.080 | 0.307 |
| Type of AF (permanent) | 0.150 | 0.033-0.682 | 0.014 |
| History of carotid disease | 0.171 | 0.052-0.561 | 0.004 |
| Male gender | 1.948 | 0.851-4.460 | 0.115 |
| HAS-BLED score | 3.613 | 1.694-7.707 | 0.001 |
| CHA2DS2-VASc score | 1.006 | 0.729-1.388 | 0.971 |
| LAAO versus NOAC | 0.419 | 0.167-1.052 | 0.064 |
AF: atrial fibrillation; CI: confidence interval; HR: hazard ratio; LAAO: left atrial appendage occlusion; NOAC: non-vitamin K anticoagulation.
Regarding the secondary endpoints in the LAAO group, there were no cardiovascular deaths. There was one recurrent stroke (1.1%) and one case of major bleeding (1.1%) that led to the patient's death. In the NOAC group, there were two cardiovascular deaths (1.3%), four recurrent strokes (2.7%) and five major bleeds (3.4%), one of which was fatal. The low number of secondary endpoint events in the LAAO group precluded any further meaningful analysis.
Left atrial appendage occlusion patients with no antithrombotic therapy after six monthsThe study's protocol required that patients undergoing LAAO complete at least six months of antiplatelet therapy. In 18.9% (n=17) of LAAO cases, antithrombotic treatment was completely discontinued six months after implantation due to recurring minor bleeds (epistaxis, bruising, or conjunctival bleeding) and/or patient request. Hence, no antithrombotic drug therapy was given to 17 of these patients after six months of follow-up. These patients were of similar age (74.7±5.5 years) to the rest of the LAAO population and had a higher prevalence of persistent AF (88.2%) and previous major bleeding (70.6%). Ischemic and bleeding risk scores were similar to the other LAAO cases (CHA2DS2-VASc 4.2±1.2, HAS-BLED 3.3±0.9) (Table 5). During follow-up (17.0±6.5 months), no deaths, stroke or major bleeding occurred in this subset of patients. Of note, follow-up transesophageal echocardiography did not demonstrate any device-related thrombus in these patients.
Baseline characteristics of left atrial appendage occlusion patients who continued antiplatelet therapy compared to those who discontinued antithrombotic treatment six months after the procedure.
| Continued antiplatelet therapy(n=74) | Discontinued antiplatelet therapy(n=17) | p | |
|---|---|---|---|
| Age, years | 74.7±9.2 | 74.7±5.5 | 0.97 |
| Male, % | 67.6 (50) | 52.9 (9) | 0.26 |
| Hypertension, % | 87.8 (65) | 82.4 (14) | 0.54 |
| Diabetes, % | 37.8 (28) | 23.5 (4) | 0.26 |
| Dyslipidemia, % | 55.4 (41) | 23.5 (4) | 0.018 |
| Congestive heart failure, % | 55.4 (41) | 52.9 (9) | 0.42 |
| Persistent AF, % | 70.3 (52) | 88.2 (15) | 0.13 |
| Previous stroke/TIA before implantation, % | 33.8 (25) | 35.3 (6) | 0.90 |
| Previous major bleeding, % | 56.8 (42) | 70.6 (12) | 0.29 |
| Mean CHA2DS2-VASc score | 4.4±1.5 | 4.2±1.2 | 0.59 |
| Mean HAS-BLED score | 3.0±1.0 | 3.3±0.9 | 0.22 |
| Renal dysfunction, % (GFR <60 ml/min) | 35.1 (26) | 23.5 (4) | 0.36 |
AF: atrial fibrillation; GFR: glomerular filtration rate; TIA: transient ischemic attack.
This is the first registry-based study assessing the role of LAAO in high-risk non-valvular AF patients in comparison with NOAC. The main finding is that LAAO does not appear to be inferior to NOAC for the prevention of a composite endpoint of all-cause death, stroke and major bleeding and is a reasonable alternative to anticoagulation.
Anticoagulation, bleeding and left atrial appendage occlusionOAC is the mainstay in the prevention of thromboembolism among patients with nonvalvular AF. Furthermore, NOAC has been shown to be at least non-inferior to warfarin for the prevention of cardioembolic events in large multicenter studies.2,7 However, stroke or systemic embolism continue to occur even in appropriately anticoagulated patients, at a rate of 1.3-1.7% per year in moderate- to high-risk cohorts.4,7 Importantly, bleeding events often occur on both warfarin and NOAC. In randomized trials comparing NOAC and warfarin, bleeding varied between 1.9 and 3.6% per year for patients on NOAC and 3.1 and 4.2% per year for those on warfarin.2,13 Furthermore, if clinically relevant bleeds are considered, the annual bleeding risk on NOAC could reach 15%.14,15,16 Although NOAC has substantially reduced the risk of intracranial hemorrhage compared to VKA, the risk of gastrointestinal bleeding is similar or even higher with selective oral anticoagulants.2,10 Alternative strategies for cardioembolic prevention are needed for those with an absolute contraindication or who are poor candidates for long-term OAC (e.g. high HAS-BLED score or prior bleed on NOAC).
Percutaneous LAAO has been tested in two randomized clinical trials, which showed at five years of follow-up that LAAO provided similar benefit to VKAs regarding ischemic stroke/systemic embolism and cardiovascular death (2.8 vs. 3.4 events per 100 patient-years, HR 0.82; p=0.27), although OAC provides benefit in reducing cerebrovascular ischemia in unrelated AF strokes.3 Regarding bleeding events, data from the PROTECT AF and PREVAIL trials demonstrated that the rate of major bleeding was significantly lower in the device group (1.0 vs. 3.5 events per 100 patient-years, HR 0.28; p<0.001) after six months, beyond the post-procedural medical regimen of aspirin and warfarin. Interestingly, bleeding events in our real-world cohort occurred at a similar rate to the PROTECT AF and PREVAIL trials, with an approximately threefold higher incidence of major bleeding in patients receiving OAC. A more recent post-hoc analysis of a pooled analysis of the randomized PROTECT-AF and PREVAIL trials,17 focusing on the overall net clinical benefit, showed that warfarin was more effective in the early phase of follow-up, but there was a trend in favor of LAAO after 1-2 years, particularly in those with prior stroke.
Data regarding the efficacy and safety of LAAO come largely from nonrandomized observational studies, which mostly compared it against VKA. While current clinical practice largely favors NOAC in AF patients, data comparing it with LAAO are lacking. No randomized trial has ever compared LAAO with NOAC. The ongoing Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in Atrial Fibrillation (PRAGUE-17) trial14 may provide further insights regarding the benefit of either treatment strategy.
Efficacy and safetyOur results suggested that LAAO was non-inferior to NOAC regarding the primary endpoint, with all events occurring at a lower incidence in LAAO patients than in those on NOAC, even in secondary prevention. Of note, while most events in the NOAC group occurred in the first year of follow-up, events reported in patients receiving LAAO were only seen after the end of the first year. In a recent network meta-analysis that included more than 87000 AF patients, the efficacy and safety of LAAO were compared with other strategies for stroke prevention.7 This study, one of few, provided an indirect assessment of NOAC versus LAAO, arguing that LAAO was superior to placebo and antiplatelet therapy, and had a similar performance to NOAC for preventing mortality, stroke and bleeding. Our study corroborated these findings in a more direct comparison. Nevertheless, the long-term clinical impact of LAAO must be weighed against the risk of procedural complications. The registry's LAAO indications and periprocedural complications were previously published elsewhere.5
High bleeding risk patients were analyzed in the ASAP study,18 in which LAAO performance was assessed in AF patients considered ineligible for warfarin (NOAC-related bleeds were not included). The authors reported a 3.8% major bleeding rate due to hematoma at the puncture site. A more comprehensive Iberian registry19 reported a bleeding rate during a two-year follow-up of 10.1%, mostly gastrointestinal (90% of cases) and related to dual or single antiplatelet therapy. Importantly, NOAC or VKAs do not seem to be safer treatment options with regards to gastrointestinal bleeding. In our study, 18.9% of LAAO patients discontinued antiplatelet therapy due to excessive minor bleeding. Although these patients had no antithrombotic therapy beyond the first six months after device implantation, no death or stroke occurred during the 24-month follow-up. Furthermore, no major bleeding occurred, suggesting LAAO as a valid option for patients at high bleeding risk.
In summary, in the appropriate setting LAAO may be a valid alternative to OAC in the form of NOAC in high-risk patients in whom long-term OAC is discouraged. Although this non-randomized study is hypothesis-generating only, it offers a valuable insight into the role of LAAO versus NOAC and provides a rationale for future randomized studies comparing the two treatment strategies.
LimitationsAlthough this is the first study to date comparing LAAO with NOAC therapy, some limitations should be taken into consideration. First, observational studies are inherently biased, and even propensity score matching cannot eliminate this bias completely. Our study should be considered as hypothesis-generating only. Second, given the low number of events, our study was ultimately underpowered to show any statistically significant differences with regards to the secondary endpoints. However, our results do suggest that LAAO was at least non-inferior to NOAC. Furthermore, three patients were lost to follow-up (Figure 1) and subsequently excluded from the final cohort. However, it is unlikely that the occurrence of any cardiovascular event in any of these cases would have had any significant impact on the study results, and there is no evidence of any change in these patients’ vital status or hospital admissions in their national vital statistics and health system records. Third, although our analysis was adjusted according to the propensity score and all mortality predictors, only about a third of LAAO patients had had a prior stroke, contrasting with the NOAC cases, who were all on secondary prevention. However, our inclusion criteria, based on the CHA2DS2-VASc score, implied that all patients included in this study were considered at high risk for stroke, and most importantly, our subgroup analysis of secondary prevention patients showed an even greater effect size. Finally, our LAAO patients received their treatment in a highly experienced center,20 and complications rates and outcomes of LAAO patients treated in less experienced hospitals may differ.
ConclusionsIn this registry-based study, LAAO was not inferior to NOAC for the prevention of a composite endpoint of all-cause mortality, stroke and major bleeding in high stroke risk patients.
FundingThe authors report no funding.
Conflicts of interestThe authors have no conflicts of interest to declare.