New-onset atrial fibrillation (AF) frequently complicates myocardial infarction, with an incidence of 6–21%.
ObjectiveTo assess the predictors and prognosis of new-onset AF during acute coronary syndromes (ACS).
MethodsWe performed a retrospective observational cohort study including 902 consecutive patients (mean age 64 years, 77.5% male) admitted to a single center over a two-year period, with a six-month follow-up.
ResultsAF rhythm was identified in 13.8% patients, of whom 73.3% presented new-onset AF and 26.8% pre-existing AF. New-onset AF was more frequent in older (p<0.001) and hypertensive patients (p=0.001) and in those with previous valvular heart disease (p<0.001) and coronary artery bypass grafting (p=0.049). During hospitalization, patients with new-onset AF more often had respiratory infection (p=0.002) and heart failure (p<0.001), and higher values of NT-proBNP (p=0.007) and peak creatinine (p=0.001). On echocardiography they had greater left atrial (LA) diameter (p<0.001) and more frequent significant mitral regurgitation (p<0.001) and left ventricular ejection fraction (LVEF) ≤40% (p<0.001) and were less likely to have significant coronary lesions (p=0.009) or to have undergone coronary revascularization (p<0.001). In multivariate analysis, age (OR 1.06, p=0.021), LVEF ≤40% (OR 4.91, p=0.002) and LA diameter (OR 1.14, p=0.008) remained independent predictors of new-onset AF. Together with age, diabetes and maximum Killip class, this arrhythmia was an independent predictor of overall mortality (OR 3.11, p=0.032).
ConclusionsAge, LVEF ≤40% and LA diameter are independent predictors of new-onset AF during ACS. This arrhythmia is associated with higher overall mortality (in-hospital and in follow-up).
A fibrilhação auricular (FA) de novo complica frequentemente o enfarte agudo do miocárdio, tendo uma incidência entre 6-21%.
ObjetivosDeterminar os preditores e prognóstico da FA de novo nas síndromes coronárias agudas (SCA).
MétodosEstudo retrospetivo observacional de coorte, incluindo 902 doentes consecutivos (idade média: 64 anos; 77,5% homens), admitidos num hospital, durante dois anos, com follow-up de seis meses.
ResultadosO ritmo de FA foi identificado em 13,8% doentes, dos quais 73,3% apresentaram FA de novo e 26,8% FA pré-existente. A FA de novo ocorreu mais nos idosos (p<0,001), hipertensos (p=0,001), doentes com história de patologia valvular (p<0,001) e cirurgia de revascularização miocárdica (p=0,049). No internamento, verificou-se maior incidência de infeção respiratória (p=0,002) e insuficiência cardíaca (p<0,001). Aferiram-se valores superiores de NT-proBNP (p=0,007) e creatinina pico (p=0,001). Na avaliação ecocardiográfica observou-se um diâmetro superior da aurícula esquerda (AE; p<0,001), maior prevalência de insuficiência mitral significativa (grau ≥ II/IV; p<0,001) e fração de ejeção ventricular esquerda ≤ 40% (FEVE ≤ 40%; p<0,001). Documentou-se ausência de lesões coronárias significativas (p=0,009) e não-revascularização coronária (p<0,001). Na análise multivariada, a idade (OR 1,06, p=0,021), a FEVE ≤ 40% (OR 4,91, p=0,002) e o diâmetro da AE (OR 1,14, p=0,008) permaneceram preditores independentes da FA de novo. Juntamente com a idade, diabetes, e classe de Killip máxima, a FA de novo foi preditora independente da mortalidade global (OR 3,11, p=0,032).
ConclusõesA idade, FEVE≤40% e diâmetro da AE são preditores independentes da FA de novo durante as SCA. Esta arritmia acarreta uma maior mortalidade global (mortalidade intra-hospitalar e durante o seguimento).
acute coronary syndromes
atrial fibrillation
left atrial
left ventricular
left ventricular ejection fraction
myocardial infarction
Atrial fibrillation (AF), the most commonly encountered clinical arrhythmia, often occurs in the setting of myocardial infarction (MI), with a reported incidence between 6 and 21%.1 New-onset AF should be differentiated from pre-existing AF, since they may have different clinical and therapeutic implications.2,3 Nevertheless, the presence of AF is associated with a high risk of heart failure and mortality in MI patients, regardless of its timing.4 During AF, rapid and irregular ventricular rates, inadequate ventricular filling and loss of atrial contribution to cardiac output lead to an increase in oxygen demand, causing further impairment in coronary perfusion and left ventricular (LV) systolic function.5 Factors precipitating AF during acute coronary syndromes (ACS) include atrial ischemia or infarction, right ventricular infarction, pericardial inflammation, acute hypoxia, ionic disturbances such as hypokalemia, hemodynamic impairment secondary to LV dysfunction, and circulating catecholamines (endogenous or exogenous).6–8 The result is a vicious circle composed of AF, myocardial ischemia and heart failure, in which each element aggravates the other two. In the early period of ACS, several factors have been associated with the occurrence of AF, such as advanced age, heart failure, LV dysfunction, mitral regurgitation, excessive sympathetic/parasympathetic nerve stimulation, pericarditis and activation of inflammation.9 It would be useful to identify predictors for the development of AF in the setting of ACS, given that identification of high-risk patients would assist in the development of prophylactic antiarrhythmic therapies.
The primary aim of this study was to identify the predictors and assess the prognosis of new-onset AF during ACS.
MethodsThis was a retrospective observational cohort study with a six-month follow-up. All patients (n=902; mean age 64 years, 77.5% male) consecutively admitted to the coronary care unit of a single center with a diagnosis of ACS between July 2009 and June 2011 were included.
Diagnoses of ACS and AF were made according to the European Society of Cardiology guidelines.10–12 Development of heart failure during hospital stay was defined as Killip class ≥2. Patients with AF were divided according to the timing of the arrhythmia: every patient who presented with AF for the first time (i.e., who did not have previously documented AF) at admission or during hospital stay was considered to have new-onset AF, while those with previously documented AF were classified as having pre-existing AF and were excluded from the statistical analysis.
Demographic, clinical, laboratory, echocardiographic and coronary angiographic data were collected. Regarding laboratory data, NT-proBNP and cystatin C values were obtained in the first 24 hours after admission, and peak creatinine was considered to be the maximum value during hospitalization. The first echocardiogram performed in hospital was used to provide echocardiographic data. Coronary angiographic data were collected from coronary angiography performed during hospital stay. Significant coronary artery disease (CAD) on coronary angiography was defined as at least one ≥50% lesion in the left main artery and/or ≥70% in other coronary arteries. Multivessel disease was defined as significant stenosis in two or more major epicardial arteries. Although this was a retrospective cohort study, all clinical and laboratory data were collected prospectively and recorded in a computerized database, in accordance with our department's protocol for patients admitted to the coronary care unit with ACS.
Statistical analysisUnivariate analysis of categorical variables was performed using the chi-square test, with results expressed as percentages, and of continuous variables using the Student's t test, with results expressed as means ± standard deviation. Multivariate logistic regression analysis was used to determine independent predictors of new-onset AF, including only variables with statistical significance on univariate analysis. Multivariate logistic analysis was also performed to determine independent predictors of overall mortality (in-hospital and follow-up). Kaplan–Meier analysis was used to illustrate six-month cumulative mortality according to the presence of new-onset AF. Differences with p<0.05 were considered significant. The statistical analysis was carried out using SPSS version 18.0.
ResultsIn the study population (n=902), AF rhythm was identified in 13.8% patients (n=124), of whom 73.3% (n=91) presented new-onset AF and 26.8% (n=33) pre-existing AF. New-onset AF patients were studied according to their baseline characteristics, in-hospital features and clinical outcomes.
Baseline characteristics and in-hospital dataThe baseline characteristics of the study population are shown in Table 1.
Baseline characteristics of the study population.
| With new-onset AF (n=91) | Without new-onset AF (n=778) | p | |
| Demographics | |||
| Age (years), mean (SD) | 71 (12) | 63 (13) | <0.001 |
| Male, % | 71.4% | 78.4% | 0.130 |
| CV risk factors, % | |||
| Hypertension | 79.1% | 62.1% | 0.001 |
| Diabetes | 29.7% | 26.5% | 0.515 |
| Dyslipidemia | 54.9% | 53.7% | 0.826 |
| Smoking | 18.7% | 31.4% | 0.013 |
| History, % | |||
| MI | 22.0% | 14.7% | 0.067 |
| CABG | 7.7% | 3.5% | 0.049 |
| VHD | 8.8% | 1.5% | <0.001 |
| Stroke | 8.8% | 7.2% | 0.582 |
| Previous medication, % | |||
| Aspirin | 36.3% | 22.9% | 0.005 |
| ACEI/ARB | 49.5% | 42.4% | 0.200 |
| Beta-blocker | 27.5% | 20.2% | 0.106 |
| Statin | 35.2% | 34.1% | 0.834 |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CABG: coronary artery bypass grafting; CV: cardiovascular; MI: myocardial infarction; SD: standard deviation; VHD: valvular heart disease.
New-onset AF was more frequent in older (p<0.001), non-smoking (p=0.013) and hypertensive (p=0.001) patients, as well as in those with previous valvular heart disease (p<0.001), history of coronary artery bypass grafting (CABG) (p=0.049) and previously treatment with aspirin (p=0.005). No differences were seen regarding other cardiovascular risk factors or history.
During hospitalization, patients with new-onset AF presented with higher heart rate (p<0.001) and had a higher prevalence of respiratory tract infections (p=0.002) and heart failure (p<0.001). Regarding laboratory parameters, higher mean NT-proBNP (p=0.007), cystatin C (p=0.033) and peak creatinine (p=0.001) were recorded. Echocardiographic data revealed an association between new-onset AF and greater left atrial (LA) diameter (p<0.001), significant mitral regurgitation (grade ≥II/IV; p<0.001) and LV ejection fraction (LVEF) ≤40% (p<0.001). These patients were also less likely to have significant coronary lesions (p=0.009) or to have undergone coronary revascularization (p<0.001), the latter due to the lower number of percutaneous coronary interventions performed (p=0.003). The presence of multivessel disease was similar in the two groups. Clinical information during hospital stay and laboratory, echocardiographic, coronary angiographic, revascularization and mortality data are summarized in Table 2.
Clinical, laboratory, echocardiographic, coronary angiographic and revascularization data and mortality (in-hospital, during follow-up and overall).
| With new-onset AF (n=91) | Without new-onset AF (n=778) | p | |
| Clinical, % | |||
| Heart rate (bpm),a mean (SD) | 85 (24) | 75 (17) | 0.001 |
| SBP (mmHg),a mean (SD) | 128 (29) | 128 (27) | 0.779 |
| Heart failure | 53.8% | 30.8% | <0.001 |
| Respiratory infection | 20.5% | 6.6% | 0.007 |
| ST-elevation ACS | 61.5% | 52.7% | 0.110 |
| Blood tests, mean (SD) | |||
| Peak creatinine (mg/dl) | 1.36 (0.85) | 1.15 (0.52) | 0.001 |
| Cystatin C (mg/l) | 1.03 (1.12) | 0.86 (0.56) | 0.033 |
| CRP (mg/l) | 23 (36) | 16 (33) | 0.104 |
| NT-proBNP (pg/ml) | 4495 (8673) | 2639 (5393) | 0.007 |
| Echocardiography | |||
| LA diameter (mm), mean (SD) | 46 (6) | 42 (5) | <0.001 |
| LVEF ≤40%, % | 53.9% | 31.0% | <0.001 |
| MR (grade >II/IV), % | 33.3% | 13.1% | <0.001 |
| Coronary angiography | |||
| Absence of significant CAD, % | 13.2% | 5.9% | 0.009 |
| Multivessel disease, % | 64.2% | 62.6% | 0.778 |
| Coronary revascularization | |||
| PCI | 49.4% | 66.0% | 0.003 |
| CABG | 12.9% | 13.7% | 0.847 |
| Total | 62.4% | 79.7% | <0.001 |
| Mortality | |||
| During hospital stay | 8.8% | 4.1% | 0.044 |
| During follow-up | 6.6% | 3.7% | 0.217 |
| Overall | 13.4% | 5.9% | 0.010 |
ACS: acute coronary syndrome; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CRP: C-reactive protein; LA: left atrial; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; NT-proBNP: N-terminal pro-brain natriuretic peptide; PCI: percutaneous coronary intervention; SBP: systolic blood pressure.
In multivariate analysis, age (odds ratio [OR] 1.06, 95% confidence interval [CI] 1.01–1.12, p=0.021), LVEF ≤40% (OR 4.91, 95% CI 1.77–13.57, p=0.002) and LA diameter (OR 1.14, 95% CI 1.03–1.25, p=0.008) remained independent predictors of new-onset AF (Table 3). Respiratory infection failed to reach statistical significance by a narrow margin (OR 4.17, 95% CI 0.99–17.38, p=0.050).
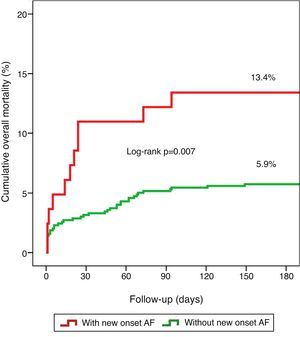
Clinical outcomes and prognosisNew-onset AF was a predictor of death (p=0.044) during hospital stay and showed a tendency towards higher mortality during follow-up (6.6% vs. 3.7%, p=0.207; mean follow-up 197±78 days). When both in-hospital and follow-up mortality were assessed collectively (overall mortality), new-onset AF was related to worse prognosis (13.4% vs. 5.9%, log rank p=0.007), as illustrated by the Kaplan–Meier curves in Figure 1.
Together with age, diabetes and maximum Killip class III and IV, new-onset AF remained an independent predictor of overall mortality, carrying a risk 3.11 times higher than in the group without AF (95% CI 1.10–8.76, p=0.032), as shown in Table 4.
Independent predictors of overall mortality.
| Variables | OR | 95% CI | p |
| Age | 1.052 | 1.003–1.104 | 0.039 |
| Diabetes | 2.690 | 1.104–6.556 | 0.030 |
| New-onset AF | 3.105 | 1.100–8.763 | 0.032 |
| Maximum Killip class II | 2.056 | 0.653–6.477 | 0.218 |
| Maximum Killip class III | 6.611 | 1.583–27.606 | 0.010 |
| Maximum Killip class IV | 17.971 | 4.346–74.320 | <0.001 |
AF: atrial fibrillation; CI: confidence interval; OR: odds ratio.
Our group reported earlier13 that in the context of ACS the presence of AF by itself, documented in the first 48 hours of hospitalization, was an independent predictor of in-hospital and six-month mortality. The relevance of AF type (pre-existing or new-onset) was not assessed. In this study we refined the definition of AF according to its timing to scrutinize the role of new-onset AF.
The present study demonstrated that: (1) age, LVEF ≤40% and LA diameter are independent predictors of new-onset AF; (2) new-onset AF is associated with worse clinical features during hospital stay; and (3) new-onset AF is associated with higher in-hospital and overall mortality and a tendency towards higher mortality during follow-up.
The incidence of new-onset AF was 10.1% in our population, which is similar to that described in the literature.1–3,14–17
In a recent review regarding new-onset AF in the setting of ACS, Lau et al.18 reported several factors associated with new-onset AF following multivariate analysis, including advanced age, higher Killip class or heart failure, hypotension, higher heart rate, history of hypertension, history of stroke, female gender, increased peak creatinine and increased C-reactive protein levels. Previous studies have also documented the association of new-onset AF with LV dysfunction.1 In our study, some of these variables were also related to the development of new-onset AF in univariate analysis (heart failure, higher heart rate, history of hypertension and increased peak creatinine), but only advanced age and LV dysfunction remained as risk factors in multivariate analysis. The mechanisms underlying the relation between AF and these factors include age-associated loss of atrial myocardium and conduction disturbances and the increased atrial pressure and volume overload, secondary valvular dysfunction and chronic neurohormonal stimulation that occur in LV dysfunction and heart failure.10
Besides the well-known risk factors described above, LA diameter remained an independent predictor for AF occurrence in our study. LA diameter, a parameter not often assessed in other studies, is a marker of progressive dilatation and remodeling of LA myocardium, which acts as a substrate for AF initiation and maintenance.19 However, this association may also be explained by the presence of other risk factors: predictors of new-onset AF in univariate analysis such as hypertension, a recognized risk factor for new-onset AF and AF-related complications,10 and valvular heart disease, a frequent finding in AF patients,10 could have contributed to LA dilatation. LA diameter is an easily obtainable parameter on the transthoracic echocardiogram that may help to stratify risk for new-onset AF in the setting of ACS.
Patients with previous CABG also had a predisposition to development of AF in univariate analysis, reinforcing the close relationship between CAD and this arrhythmia. Aspirin use was a predictor in univariate analysis, possibly because it is frequently prescribed in patients with documented CAD or at risk for CAD.
Respiratory infection was a risk factor in univariate analysis and almost reached statistical significance in multivariate analysis. This association has been described as a predictor of AF after CABG.20 Inflammation and active infection promote the release of cytokines and upregulation of Toll-like receptor-2 expression on monocytes, which may act as a trigger for AF.21
During hospital stay, new-onset AF was associated with worse laboratory and echocardiographic features. Elevated biomarkers associated with renal dysfunction (peak creatinine and cystatin C) and with heart failure (NT-proBNP) were recorded. Besides the echocardiographic parameters (LVEF ≤40% and LA diameter) described above, significant mitral regurgitation (grade >II/IV) predicted new-onset AF in univariate analysis. It is known that significant mitral regurgitation (from degenerative causes or organic valvular abnormalities), through increase in LA size, is associated with development of AF, at a rate of about 5% per year.22
Coronary angiography revealed an association between new-onset AF and the absence of coronary lesions and lower rates of revascularization. The former could be a consequence of early reperfusion after a thromboembolic event or simply the result of an imbalance between myocardial oxygen supply and demand caused by the tachyarrhythmia.11 The latter, due to a lower rate of percutaneous coronary intervention (CABG remained unchanged), a finding also reported by other authors,18 might also be secondary to the hypotheses mentioned above, but could additionally be associated with optimal medical treatment choice due to severe comorbidities or diffuse CAD not amenable to revascularization.
Our analysis of new-onset AF in the setting of ACS showed that, as in previous publications,14–18 these patients had higher overall (in-hospital plus follow-up) mortality (13.4% vs. 5.9%, log rank p=0.007). As described by McManus et al.23 in the large multinational GRACE registry including 59 032 patients hospitalized with ACS, new-onset AF was associated with high overall in-hospital mortality (14.5%). However, in the latter study short-term mortality declined over the study period (between 2000 and 2007), likely reflecting enhanced treatment. Improvements in ACS therapy in the last decade may also explain why we found lower in-hospital mortality in new-onset AF patients (8.8%). The relation between new-onset AF and worse prognosis could be explained as a function of the comorbidities associated with this arrhythmia, since young patients with AF and no structural heart disease do not have increased mortality.24 However, new-onset AF remained an independent predictor of overall mortality in multivariate analysis, and the literature indicates that AF is an independent powerful adverse prognostic factor.4,14–18,25 Although not addressed by this study, several other questions should be considered regarding the setting of new-onset AF and ACS. During ACS, the clinical impact and prognosis of AF type (new-onset or pre-existing) may be different, but the published data are not consensual.2–4,26,27 How should new-onset AF in this context be treated and prevented? In terms of long-term management, what therapeutic strategy (rate control vs. rhythm management) should be preferred? Do antiarrhythmic drugs have a role to play? Should all patients be discharged under anticoagulation therapy?
The literature in these fields is scarce, especially concerning optimal treatment modalities. Randomized controlled trials are needed to clarify these issues.
LimitationsThere are several limitations to be considered in the interpretation of our study. First, this was a retrospective, observational and non-randomized study conducted at a single hospital, and as such, both identified and unidentified confounders may have influenced the outcomes. For instance, the duration of new-onset AF could not be determined with certainty in patients without AF history who presented with this arrhythmia at admission, and they may therefore have been misclassified. Indeed, patients with AF of different timing may have different clinical features and prognosis. Second, the number of patients studied with new-onset AF was small, which could limit the number of independent predictors identified and the consistency of the results. Third, we did not investigate other potential risk factors described in the literature, such as diastolic function.28 Fourth, most variables were determined by consulting medical records that could have been incomplete. Finally, as stated above, several other questions remain unanswered, especially those regarding the effect of AF type and therapeutic management in the short and long term.
ConclusionsPatients with new-onset AF in the context of ACS have worse clinical manifestations during hospitalization and adverse prognostic implications not only for the period of hospital stay but also throughout follow-up. Age, LV systolic dysfunction (LVEF ≤40%) and greater LA diameter are risk factors for new-onset AF.
Better data are needed to enable the development of strategies to stratify and treat these patients in the short and long term.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestsThe authors have no conflicts of interests to declare.