Given the increasing focus on early mortality and readmission rates among patients with acute coronary syndrome (ACS), this study was designed to evaluate the accuracy of the GRACE risk score for identifying patients at high risk of 30-day post-discharge mortality and cardiovascular readmission.
MethodsThis was a retrospective study carried out in a single center with 4229 ACS patients discharged between 2004 and 2010. The study endpoint was the combination of 30-day post-discharge mortality and readmission due to reinfarction, heart failure or stroke.
ResultsOne hundred and fourteen patients had 30-day events: 0.7% mortality, 1% reinfarction, 1.3% heart failure, and 0.2% stroke. After multivariate analysis, the six-month GRACE risk score was associated with an increased risk of 30-day events (HR 1.03, 95% CI 1.02–1.04; p<0.001), demonstrating good discrimination (C-statistic: 0.79±0.02) and optimal fit (Hosmer–Lemeshow p=0.83). The sensitivity and specificity were adequate (78.1% and 63.3%, respectively), and negative predictive value was excellent (99.1%). In separate analyses for each event of interest (all-cause mortality, reinfarction, heart failure and stroke), assessment of the six-month GRACE risk score also demonstrated good discrimination and fit, as well as adequate predictive values.
ConclusionsThe six-month GRACE risk score is a useful tool to predict 30-day post-discharge death and early cardiovascular readmission. Clinicians may find it simple to use with the online and mobile app score calculator and applicable to clinical daily practice.
Tendo em conta a importância crescente das taxas de mortalidade e readmissão precoce nos doentes com síndrome coronária aguda (SCA), realizámos este estudo que pretende avaliar a precisão do score de risco GRACE na identificação dos doentes com risco elevado de readmissão e mortalidade cardiovascular no primeiro mês após a alta.
MétodoEstudo retrospetivo efetuado num único centro com 4229 doentes com SCA com alta entre 2004-2010. Objetivo primário foi a combinação de mortalidade e readmissão por reenfarte, insuficiência cardíaca ou acidente vascular cerebral aos 30 dias após a alta.
ResultadosCento e catorze doentes tiveram eventos aos 30 dias: mortalidade 2,7%; reenfarte 1%; insuficiência cardíaca 1,3%; e acidente vascular cerebral 0,2%. Após uma análise multivariada, o score de risco GRACE aos seis meses esteve associado com um maior risco de eventos aos 30 dias (HR 1,03, IC 95% 1,02-1,04, p<0,001), demonstrando uma boa discriminação (C-statistics: 0,79±0,02) com uma calibração ótima (HL p: 0,83). A sensibilidade e especificidade foram adequadas (78,1-63,3%, respetivamente), com um valor preditivo negativo excelente (99,1%). Numa análise separada de cada um dos eventos em causa (mortalidade por todas as causas, reenfarte, insuficiência cardíaca ou acidente vascular cerebral), a avaliação do score de risco GRACE aos seis meses mostrou também uma boa discriminação e calibração, assim como valores preditivos adequados.
ConclusõesO score de risco GRACE aos seis meses é um instrumento útil na predição da morte e das readmissões precoces cardiovasculares aos 30 dias. Os médicos podem recorrer facilmente a este score (app móvel, online) e aplicá-lo na prática clínica diária.
Acute coronary syndrome (ACS) is a high-risk condition and a common cause of hospital admission around the world. Hospitalization for ACS and its early aftermath define a period of vulnerability, during which clinical deterioration leads to readmission. Since readmission after an ACS is common, expensive, and varies across hospitals, suggesting preventable events, national health systems have identified readmission as an opportunity to improve quality of care and reduce costs.1 In this context, the transition of care from the inpatient to the outpatient setting is currently seen as an opportunity to prevent readmission.2
To improve efficiency, the highest intensity interventions should target the patients who are most likely to benefit.3 Against this background, the purposes of this study were to determine the significant predictors of 30-day mortality and early cardiovascular morbidity following discharge after an ACS, and to evaluate the utility of the Global Registry of Acute Coronary Events (GRACE) risk score in this setting.
MethodsData sources and samplesThis was a retrospective study in which demographic, clinical, and angiographic data, as well as data on management and in-hospital complications, had been prospectively collected and recorded in an electronic database. Subjects were all patients with a diagnosis of ACS admitted consecutively to our hospital between January 2004 and June 2010. The initial cohort consisted of 4645 patients, of whom 274 died during the in-hospital phase. Of the 4371 discharged patients, those in whom ACS was triggered in the context of surgery, sepsis, trauma, or cocaine consumption (n=41), and those missing data for any variable of the GRACE risk score (n=67), were excluded. Of the remaining 4263 patients, one-month follow-up was completed in 99.2% (34 patients without follow-up data). Thus, the final cohort was composed of 4229 patients. The study complies with the Declaration of Helsinki, and was approved by the Clinical Research Ethics Committee of our hospital.
DefinitionsThe diagnosis of ACS was validated, through retrospective chart review, if the patient had new onset symptoms suggestive of myocardial ischemia and any of the following criteria: cardiac biomarkers above the upper limit of normal, ST-segment deviation on electrocardiogram, in-hospital stress testing showing ischemia, or known history of coronary artery disease. Patients were classified as having ST-elevation myocardial infarction (STEMI) or non-ST elevation ACS (NSTE-ACS) (non-ST elevation myocardial infarction or unstable angina). The diagnosis of unstable angina was based on suggestive symptoms together with objective evidence of myocardial ischemia on stress testing or detection of a ≥50% culprit lesion on coronary angiography, in addition to cardiac biomarkers below the upper normal limit. Left ventricular ejection fraction (LVEF) was determined by two-dimensional echocardiography. Depressed LVEF was defined as values ≤40%. In accordance with the World Health Organization criteria, anemia was defined as hemoglobin concentration below normal (<13 g/dl in men and <12 g/dl in women). Occurrence and severity of in-hospital bleeding were recorded using the Thrombolysis In Myocardial Infarction (TIMI) scheme.4 Major bleeding was defined as intracerebral hemorrhage or clinically overt bleeding associated with a drop in hemoglobin of ≥5 g/dl, while minor bleeding was defined as a drop in hemoglobin of 3–5 g/dl. In our study, severe bleeding was defined as TIMI major or minor bleeding unrelated to coronary artery bypass grafting.
Study endpointThe study endpoint was the combination of 30-day post-discharge mortality and readmission due to reinfarction, heart failure or stroke. All patients were followed for 30 days or until any event involving the combined endpoint. Follow-up methods involved one or more of the following: use of hospital records, hospital visits, telephone call to the patient's general physician, and telephone call to the patient.
GRACE risk score calculationThe two versions of the GRACE risk score (in-hospital and six-month) were calculated for each patient from the sum of the individual scores assigned to each of the corresponding variables, as previously described5,6; thus, the sum of these scores corresponded to the total GRACE risk score as a continuous variable. In addition, patients were categorized in different risk groups according to cutoff points and intervals established by the GRACE risk score. Accordingly, three risk categories (low, intermediate and high) were established.
Statistical analysisAll analyses were performed using SPSS (version 17.0, SPSS Inc., Chicago, IL). Continuous variables were described as mean ± standard deviation (SD) or as median and interquartile range. The Student's t test or Mann–Whitney U test, as appropriate, were used for comparisons of continuous variables between two groups of patients. Discrete variables were expressed as frequencies and percentages, and were compared with the chi-square test. A parsimonious logistic regression model was used to estimate the odds ratios (OR) and 95% confidence intervals (CI), assessing the performance of the GRACE risk score to predict 30-day events in a multivariate model. In the initial model variables that resulted in significant predictors of 30-day events in the univariate model were included. Multicollinearity was assessed by examining pairwise correlations between all continuous predictor variables and by assessing the variance inflation factor for each predictor variable. The contribution of each significant predictor in the multivariate model was ranked by its F-value, and variables with the smallest contribution to the model were sequentially eliminated. The final model was composed of 11 variables: age, diabetes, LVEF ≤40%, grade 3–4 mitral regurgitation, in-hospital infection, anemia at discharge, absence of coronary stenosis, percutaneous coronary intervention (PCI) with drug-eluting stents, beta-blockers at discharge, statins at discharge, and six-month GRACE risk score. A p value <0.05 was considered statistically significant.
Measures of fit and discrimination were used to assess performance of the GRACE risk score, including the Hosmer–Lemeshow (HL) goodness-of-fit test,7 in which higher p values indicate better fit. The GRACE risk score was entered into a logistic regression model to generate the individual probability of death. The HL statistic from the regression modeling was used as an indicator of the goodness-of-fit of the GRACE risk score as an overall predictor variable. Discriminatory power was assessed by the C-statistic, equivalent to the area under the receiver-operating characteristic curve.8 Negative and positive predictive values for the GRACE risk score were also computed for the high-risk group.
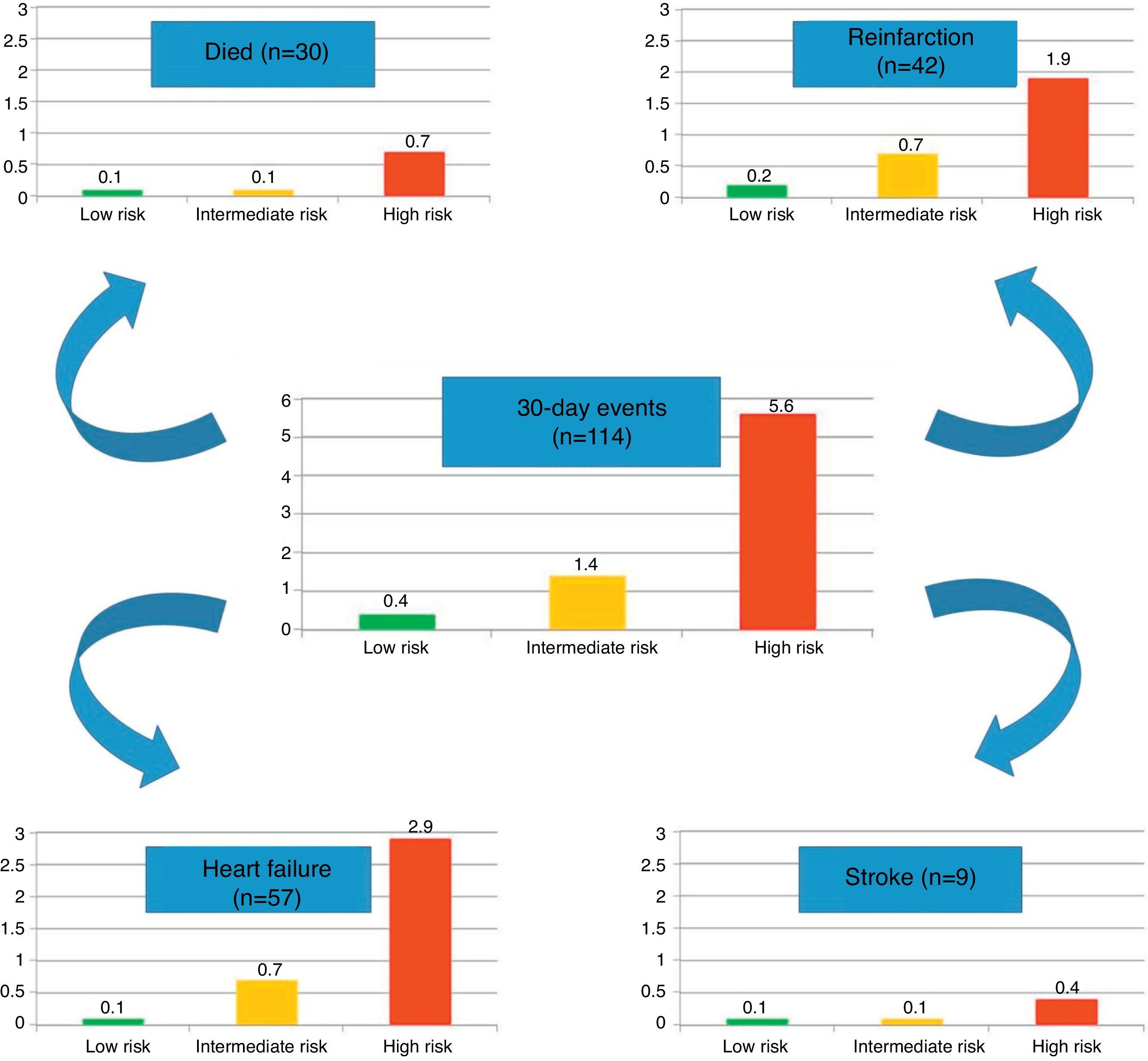
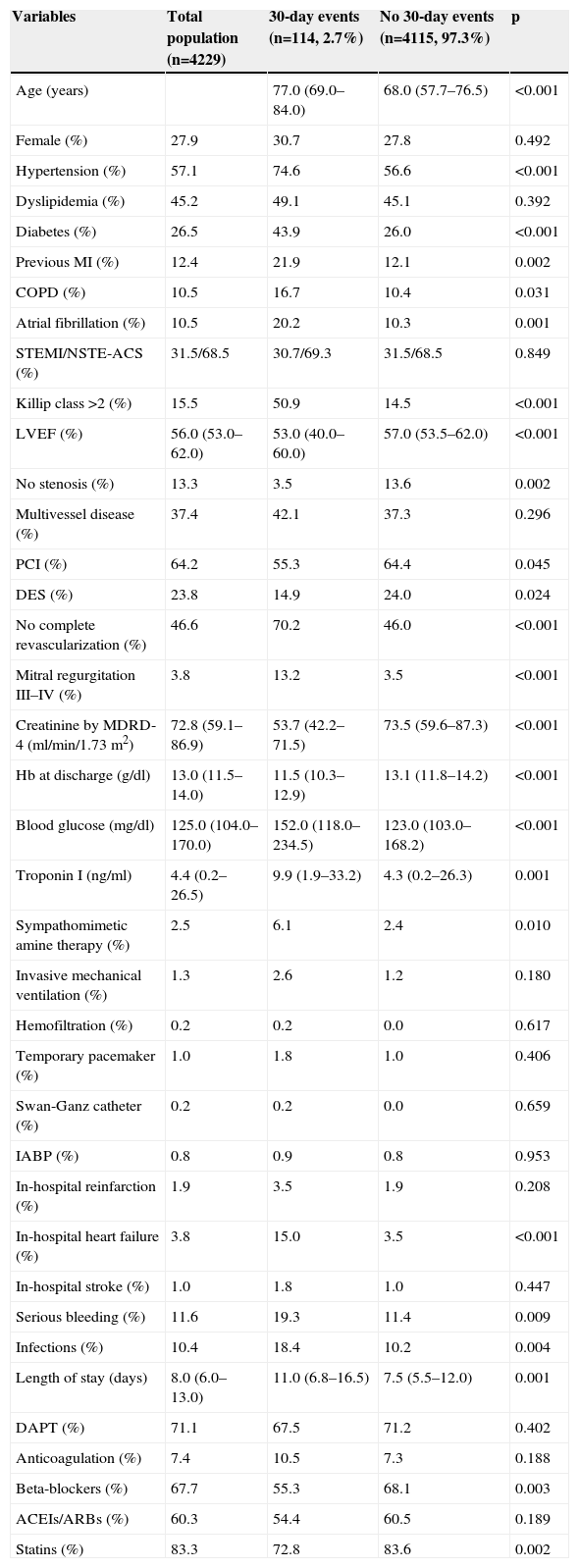
ResultsBaseline characteristics and eventsDemographic, clinical and procedural characteristics are shown in Table 1, comparing groups of patients with and without 30-day events. Overall, 2.7% of patients presented events within 30 days of discharge: 30 died (0.7%), 42 had reinfarction (1.0%), 57 were admitted for heart failure (1.3%), and nine had a stroke (0.2%).
Demographic and clinical characteristics stratified by 30-day event status.
| Variables | Total population (n=4229) | 30-day events (n=114, 2.7%) | No 30-day events (n=4115, 97.3%) | p |
|---|---|---|---|---|
| Age (years) | 77.0 (69.0–84.0) | 68.0 (57.7–76.5) | <0.001 | |
| Female (%) | 27.9 | 30.7 | 27.8 | 0.492 |
| Hypertension (%) | 57.1 | 74.6 | 56.6 | <0.001 |
| Dyslipidemia (%) | 45.2 | 49.1 | 45.1 | 0.392 |
| Diabetes (%) | 26.5 | 43.9 | 26.0 | <0.001 |
| Previous MI (%) | 12.4 | 21.9 | 12.1 | 0.002 |
| COPD (%) | 10.5 | 16.7 | 10.4 | 0.031 |
| Atrial fibrillation (%) | 10.5 | 20.2 | 10.3 | 0.001 |
| STEMI/NSTE-ACS (%) | 31.5/68.5 | 30.7/69.3 | 31.5/68.5 | 0.849 |
| Killip class >2 (%) | 15.5 | 50.9 | 14.5 | <0.001 |
| LVEF (%) | 56.0 (53.0–62.0) | 53.0 (40.0–60.0) | 57.0 (53.5–62.0) | <0.001 |
| No stenosis (%) | 13.3 | 3.5 | 13.6 | 0.002 |
| Multivessel disease (%) | 37.4 | 42.1 | 37.3 | 0.296 |
| PCI (%) | 64.2 | 55.3 | 64.4 | 0.045 |
| DES (%) | 23.8 | 14.9 | 24.0 | 0.024 |
| No complete revascularization (%) | 46.6 | 70.2 | 46.0 | <0.001 |
| Mitral regurgitation III–IV (%) | 3.8 | 13.2 | 3.5 | <0.001 |
| Creatinine by MDRD-4 (ml/min/1.73 m2) | 72.8 (59.1–86.9) | 53.7 (42.2–71.5) | 73.5 (59.6–87.3) | <0.001 |
| Hb at discharge (g/dl) | 13.0 (11.5–14.0) | 11.5 (10.3–12.9) | 13.1 (11.8–14.2) | <0.001 |
| Blood glucose (mg/dl) | 125.0 (104.0–170.0) | 152.0 (118.0–234.5) | 123.0 (103.0–168.2) | <0.001 |
| Troponin I (ng/ml) | 4.4 (0.2–26.5) | 9.9 (1.9–33.2) | 4.3 (0.2–26.3) | 0.001 |
| Sympathomimetic amine therapy (%) | 2.5 | 6.1 | 2.4 | 0.010 |
| Invasive mechanical ventilation (%) | 1.3 | 2.6 | 1.2 | 0.180 |
| Hemofiltration (%) | 0.2 | 0.2 | 0.0 | 0.617 |
| Temporary pacemaker (%) | 1.0 | 1.8 | 1.0 | 0.406 |
| Swan-Ganz catheter (%) | 0.2 | 0.2 | 0.0 | 0.659 |
| IABP (%) | 0.8 | 0.9 | 0.8 | 0.953 |
| In-hospital reinfarction (%) | 1.9 | 3.5 | 1.9 | 0.208 |
| In-hospital heart failure (%) | 3.8 | 15.0 | 3.5 | <0.001 |
| In-hospital stroke (%) | 1.0 | 1.8 | 1.0 | 0.447 |
| Serious bleeding (%) | 11.6 | 19.3 | 11.4 | 0.009 |
| Infections (%) | 10.4 | 18.4 | 10.2 | 0.004 |
| Length of stay (days) | 8.0 (6.0–13.0) | 11.0 (6.8–16.5) | 7.5 (5.5–12.0) | 0.001 |
| DAPT (%) | 71.1 | 67.5 | 71.2 | 0.402 |
| Anticoagulation (%) | 7.4 | 10.5 | 7.3 | 0.188 |
| Beta-blockers (%) | 67.7 | 55.3 | 68.1 | 0.003 |
| ACEIs/ARBs (%) | 60.3 | 54.4 | 60.5 | 0.189 |
| Statins (%) | 83.3 | 72.8 | 83.6 | 0.002 |
ACEIs/ARBs: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; COPD: chronic obstructive pulmonary disease; DAPT: dual antiplatelet therapy; DES: drug-eluting stent; Hb: hemoglobin; IABP: intra-aortic balloon pump; LVEF: left ventricular ejection fraction; MDRD-4: 4-variable Modification of Diet in Renal Disease equation; MI: myocardial infarction; NSTE-ACS: non-ST-segment elevation ACS; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
* Results expressed as percentage or median and interquartile range.
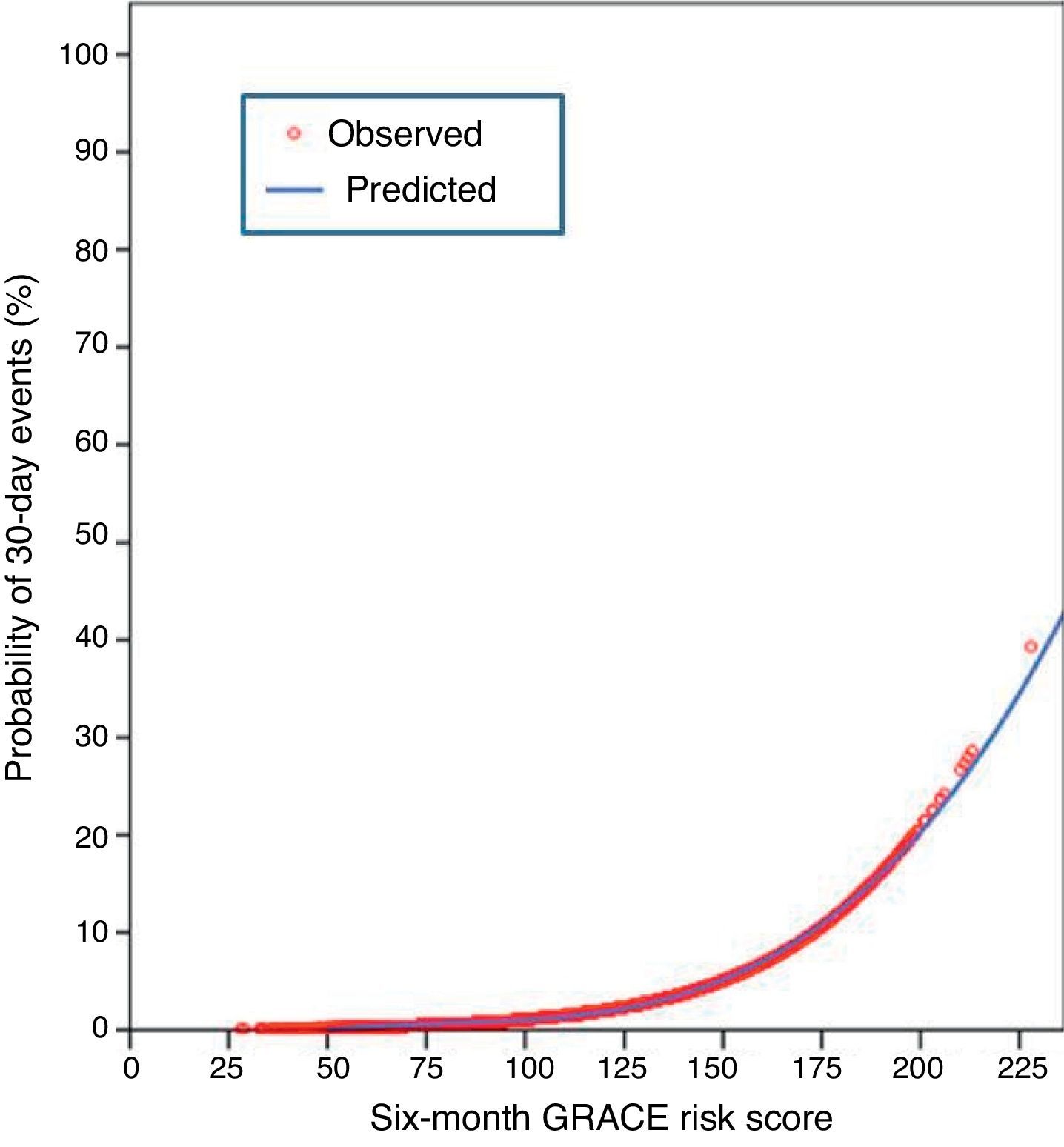
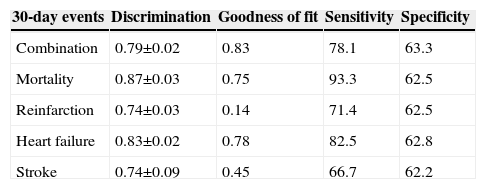
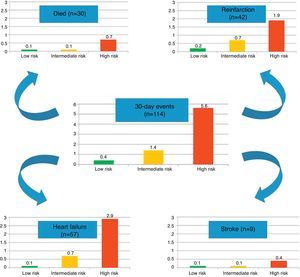
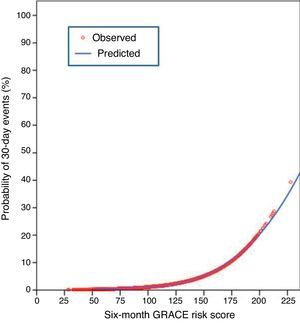
In the present cohort, the mean six-month GRACE score was 112.9±33.4. Using the mortality risk stratification proposed for ACS, 29.4% (n=1245) of patients were classified as low risk, 32.7% (n=1383) as intermediate risk, and 37.9% (n=1601) as high risk. The GRACE score stratification (low, intermediate and high) was correlated with a progressive increase in 30-day mortality, reinfarction, heart failure and stroke (Figure 1). The sensitivity and specificity of the high risk GRACE classification for 30-day events were 78.1% (95% CI 69.2%–85.1%) and 63.3% (61.8%–64.7%), respectively. Although the positive predictive value was low (5.6%, 95% CI 4.5%–6.8%), with a high false positive rate, the negative predictive value was extremely high (99.1%, 95% CI 98.6%–99.4%). As a continuous variable, a higher six-month GRACE score was found in the group that reached the study endpoint of events within 30 days of discharge (148.4±30.4 vs. 111.9±33.0, p<0.001), and in the subgroups of 30-day mortality (161.1±26.2 vs. 112.6±33.2, p<0.001), reinfarction (141.7±30.8 vs. 112.6±33.3, p<0.001), heart failure (153.6±27.6 vs. 112.4±33.2, p<0.001), and stroke (144.8±39.8 vs. 112.9±33.4; p=0.004) (Table 2). Assessment of the fit and overall performance of the GRACE score demonstrated a good fit (p=0.83 for the HL goodness-of-fit test) and high discriminatory power (C-statistic: 0.79±0.02) for the combined endpoint and for each event separately (Table 3). The fit and discrimination of the GRACE risk score were also good in both the PCI and non-PCI groups, with HL p values of 0.849 and 0.765, and C-statistics of 0.78±0.03 and 0.80±0.03, respectively. In Figure 2 a continuous model of the interaction between GRACE risk score and probability of 30-day events is shown.
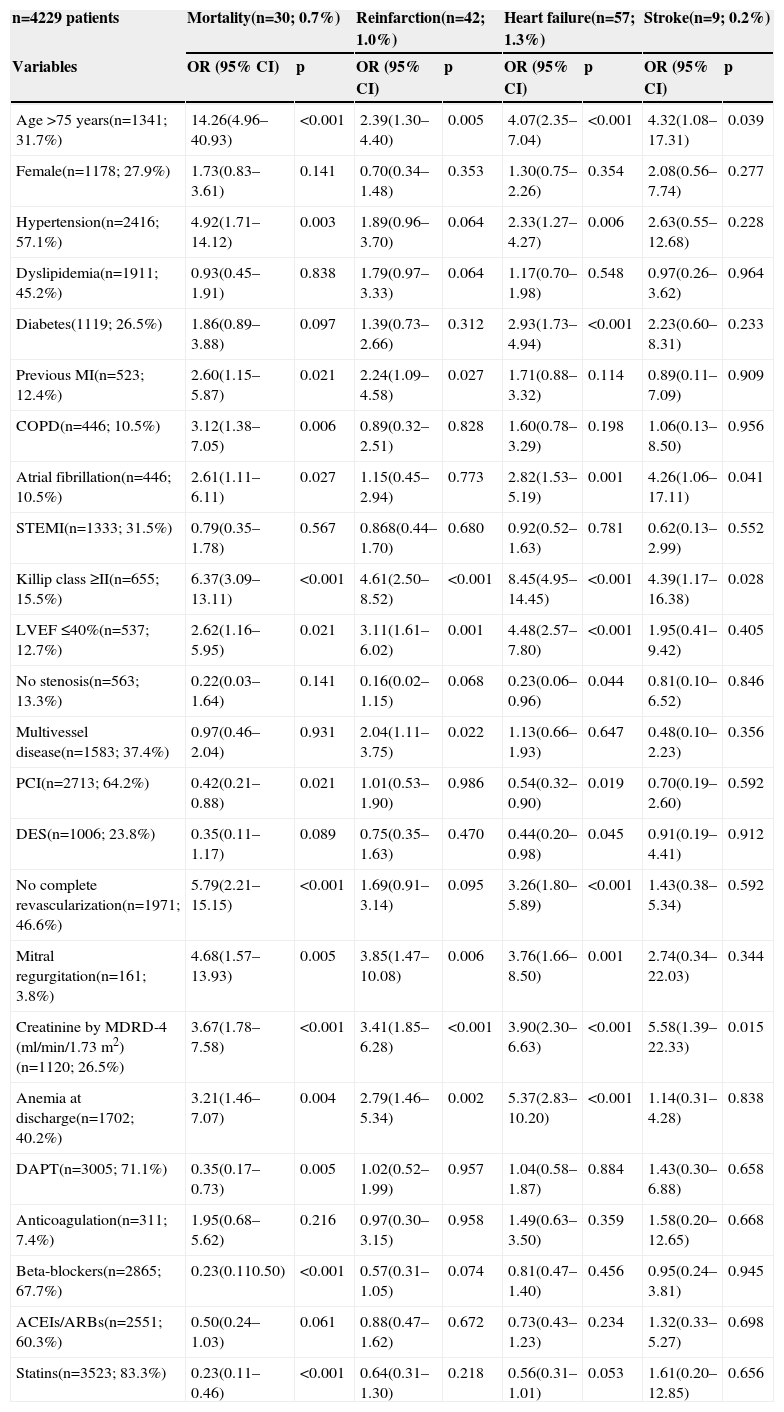
Univariate analysis for prediction of each 30-day event.
| n=4229 patients | Mortality(n=30; 0.7%) | Reinfarction(n=42; 1.0%) | Heart failure(n=57; 1.3%) | Stroke(n=9; 0.2%) | ||||
|---|---|---|---|---|---|---|---|---|
| Variables | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Age >75 years(n=1341; 31.7%) | 14.26(4.96–40.93) | <0.001 | 2.39(1.30–4.40) | 0.005 | 4.07(2.35–7.04) | <0.001 | 4.32(1.08–17.31) | 0.039 |
| Female(n=1178; 27.9%) | 1.73(0.83–3.61) | 0.141 | 0.70(0.34–1.48) | 0.353 | 1.30(0.75–2.26) | 0.354 | 2.08(0.56–7.74) | 0.277 |
| Hypertension(n=2416; 57.1%) | 4.92(1.71–14.12) | 0.003 | 1.89(0.96–3.70) | 0.064 | 2.33(1.27–4.27) | 0.006 | 2.63(0.55–12.68) | 0.228 |
| Dyslipidemia(n=1911; 45.2%) | 0.93(0.45–1.91) | 0.838 | 1.79(0.97–3.33) | 0.064 | 1.17(0.70–1.98) | 0.548 | 0.97(0.26–3.62) | 0.964 |
| Diabetes(1119; 26.5%) | 1.86(0.89–3.88) | 0.097 | 1.39(0.73–2.66) | 0.312 | 2.93(1.73–4.94) | <0.001 | 2.23(0.60–8.31) | 0.233 |
| Previous MI(n=523; 12.4%) | 2.60(1.15–5.87) | 0.021 | 2.24(1.09–4.58) | 0.027 | 1.71(0.88–3.32) | 0.114 | 0.89(0.11–7.09) | 0.909 |
| COPD(n=446; 10.5%) | 3.12(1.38–7.05) | 0.006 | 0.89(0.32–2.51) | 0.828 | 1.60(0.78–3.29) | 0.198 | 1.06(0.13–8.50) | 0.956 |
| Atrial fibrillation(n=446; 10.5%) | 2.61(1.11–6.11) | 0.027 | 1.15(0.45–2.94) | 0.773 | 2.82(1.53–5.19) | 0.001 | 4.26(1.06–17.11) | 0.041 |
| STEMI(n=1333; 31.5%) | 0.79(0.35–1.78) | 0.567 | 0.868(0.44–1.70) | 0.680 | 0.92(0.52–1.63) | 0.781 | 0.62(0.13–2.99) | 0.552 |
| Killip class ≥II(n=655; 15.5%) | 6.37(3.09–13.11) | <0.001 | 4.61(2.50–8.52) | <0.001 | 8.45(4.95–14.45) | <0.001 | 4.39(1.17–16.38) | 0.028 |
| LVEF ≤40%(n=537; 12.7%) | 2.62(1.16–5.95) | 0.021 | 3.11(1.61–6.02) | 0.001 | 4.48(2.57–7.80) | <0.001 | 1.95(0.41–9.42) | 0.405 |
| No stenosis(n=563; 13.3%) | 0.22(0.03–1.64) | 0.141 | 0.16(0.02–1.15) | 0.068 | 0.23(0.06–0.96) | 0.044 | 0.81(0.10–6.52) | 0.846 |
| Multivessel disease(n=1583; 37.4%) | 0.97(0.46–2.04) | 0.931 | 2.04(1.11–3.75) | 0.022 | 1.13(0.66–1.93) | 0.647 | 0.48(0.10–2.23) | 0.356 |
| PCI(n=2713; 64.2%) | 0.42(0.21–0.88) | 0.021 | 1.01(0.53–1.90) | 0.986 | 0.54(0.32–0.90) | 0.019 | 0.70(0.19–2.60) | 0.592 |
| DES(n=1006; 23.8%) | 0.35(0.11–1.17) | 0.089 | 0.75(0.35–1.63) | 0.470 | 0.44(0.20–0.98) | 0.045 | 0.91(0.19–4.41) | 0.912 |
| No complete revascularization(n=1971; 46.6%) | 5.79(2.21–15.15) | <0.001 | 1.69(0.91–3.14) | 0.095 | 3.26(1.80–5.89) | <0.001 | 1.43(0.38–5.34) | 0.592 |
| Mitral regurgitation(n=161; 3.8%) | 4.68(1.57–13.93) | 0.005 | 3.85(1.47–10.08) | 0.006 | 3.76(1.66–8.50) | 0.001 | 2.74(0.34–22.03) | 0.344 |
| Creatinine by MDRD-4 (ml/min/1.73 m2)(n=1120; 26.5%) | 3.67(1.78–7.58) | <0.001 | 3.41(1.85–6.28) | <0.001 | 3.90(2.30–6.63) | <0.001 | 5.58(1.39–22.33) | 0.015 |
| Anemia at discharge(n=1702; 40.2%) | 3.21(1.46–7.07) | 0.004 | 2.79(1.46–5.34) | 0.002 | 5.37(2.83–10.20) | <0.001 | 1.14(0.31–4.28) | 0.838 |
| DAPT(n=3005; 71.1%) | 0.35(0.17–0.73) | 0.005 | 1.02(0.52–1.99) | 0.957 | 1.04(0.58–1.87) | 0.884 | 1.43(0.30–6.88) | 0.658 |
| Anticoagulation(n=311; 7.4%) | 1.95(0.68–5.62) | 0.216 | 0.97(0.30–3.15) | 0.958 | 1.49(0.63–3.50) | 0.359 | 1.58(0.20–12.65) | 0.668 |
| Beta-blockers(n=2865; 67.7%) | 0.23(0.110.50) | <0.001 | 0.57(0.31–1.05) | 0.074 | 0.81(0.47–1.40) | 0.456 | 0.95(0.24–3.81) | 0.945 |
| ACEIs/ARBs(n=2551; 60.3%) | 0.50(0.24–1.03) | 0.061 | 0.88(0.47–1.62) | 0.672 | 0.73(0.43–1.23) | 0.234 | 1.32(0.33–5.27) | 0.698 |
| Statins(n=3523; 83.3%) | 0.23(0.11–0.46) | <0.001 | 0.64(0.31–1.30) | 0.218 | 0.56(0.31–1.01) | 0.053 | 1.61(0.20–12.85) | 0.656 |
Abbreviations as in Table 1.
Ability of the GRACE risk score to predict 30-day events (death, reinfarction, heart failure, stroke, and the combination).
| 30-day events | Discrimination | Goodness of fit | Sensitivity | Specificity |
|---|---|---|---|---|
| Combination | 0.79±0.02 | 0.83 | 78.1 | 63.3 |
| Mortality | 0.87±0.03 | 0.75 | 93.3 | 62.5 |
| Reinfarction | 0.74±0.03 | 0.14 | 71.4 | 62.5 |
| Heart failure | 0.83±0.02 | 0.78 | 82.5 | 62.8 |
| Stroke | 0.74±0.09 | 0.45 | 66.7 | 62.2 |
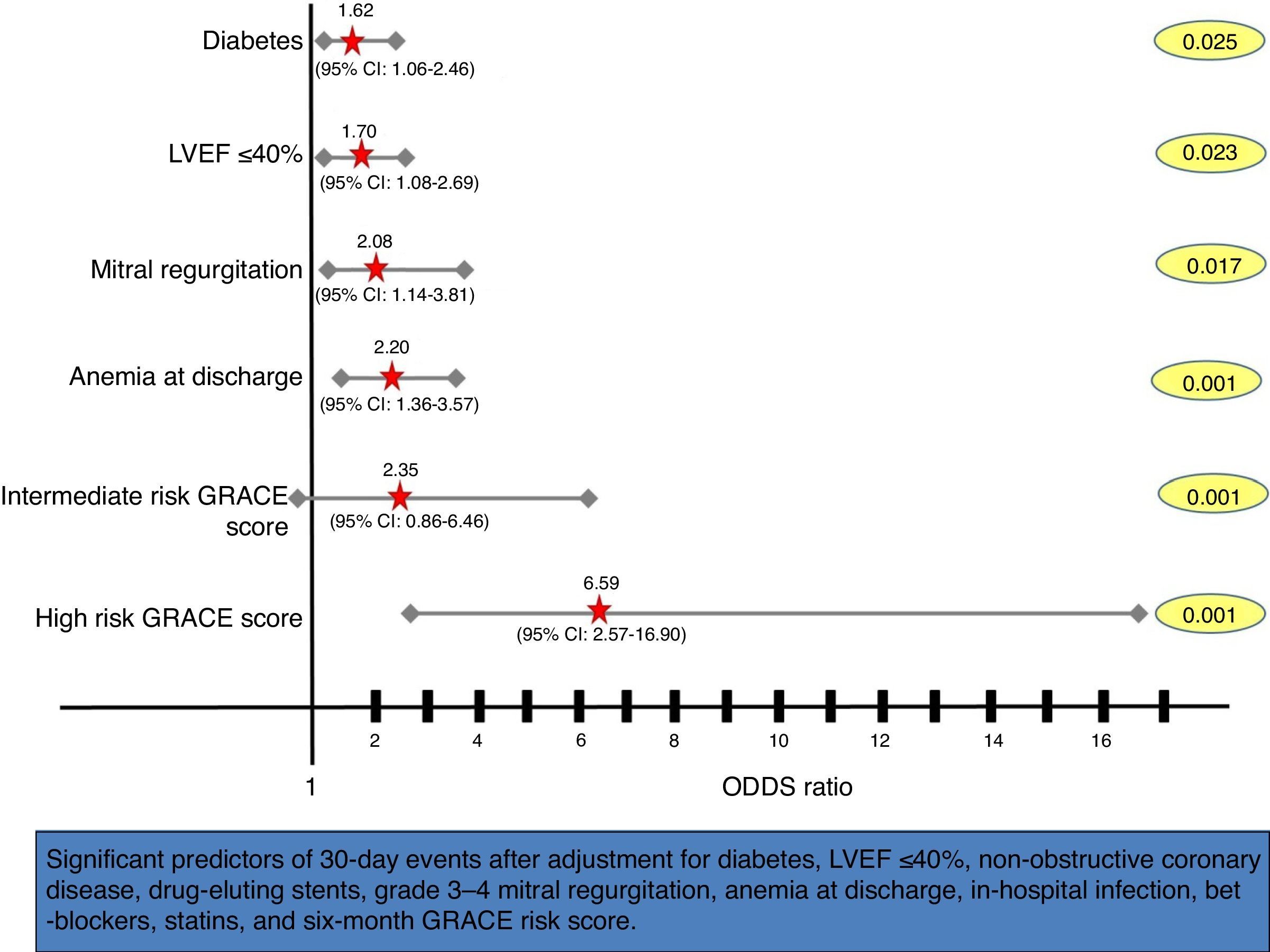
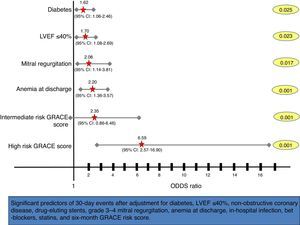
After multivariate analysis, the six-month GRACE risk score was shown to be an independent predictor of early events within 30 days of ACS (OR as a continuous variable 1.02, 95% CI 1.01–1.03; p<0.001), with a three-fold increased risk of 30-day events in the high risk GRACE group (Figure 3).
Forest plot showing multivariate predictors of 30-day events (death, reinfarction, heart failure or stroke). Adjusted odds ratios, 95% confidence intervals (CI), and p values for each variable derived from multivariate logistic regression analyses are shown. LVEF: left ventricular ejection fraction.
Given the increasing focus on 30-day readmission rates among patients with common medical conditions, including myocardial infarction, we performed this analysis in a large single-center European registry of ACS patients to determine predictors of all-cause 30-day mortality and early readmission due to cardiovascular events. We report for the first time the accuracy of the six-month GRACE risk score to predict 30-day post-discharge death and cardiovascular events (reinfarction, heart failure and stroke).
In the PCI era, survival to hospital discharge has improved dramatically.9 However, patients who do survive to discharge are at risk for post-discharge readmission. Early readmission rates have been proposed as quality measures for hospitals, particularly in the USA.10 Therefore, to improve quality and reduce costs, policy markers are increasingly focusing on 30-day readmission rates for ACS as both a quality and an economic measure. Being able to identify high-risk patients should be useful to clinicians and hospitals in stratifying patients on the basis of readmission risk and potentially as a basis for interventions to reduce readmission rates in high-risk patients. Several studies examining predictors of early readmission have recently been published2,3,10–18; however, few variables were consistently identified. Thus, clinically, early risk stratification is challenging. From a clinical perspective, there is currently no validated risk-standardized model available in the literature to identify high-risk ACS patients based on 30-day mortality and early readmission rates. Our study is an important addition to the evolving body of knowledge regarding early post-discharge mortality and readmissions. We confirmed some risk factors for discharge, but, in addition, we identified the six-month GRACE risk score as a new independent predictor of early mortality and readmission due to cardiovascular events. In our view, the six-month GRACE risk score may be useful to clinicians, hospital administrators, and investigators designing interventions to reduce early events and readmissions after ACS.
Despite the proven utility of risk scores in prognostication and guidance of treatment strategies, it is not known how often they are actually used in routine practice.19 Some physicians may be reluctant to use risk scores at the bedside because they find them inconvenient and time-consuming. Others believe that they can readily discern and integrate high-risk features into overall risk estimation without the aid of risk scores. In recent years, definitive data have demonstrated the incremental prognostic utility of risk scores beyond overall risk assessment by physicians.20,21 Although there are numerous established prognostic markers, they usually co-exist and their importance hinges on the inter-relationship of many factors. Because patients often present with complex risk profiles, assimilation of all the relevant information from history, physical examination, and laboratory investigations is a highly complicated process and a daunting task for a busy clinician.19 In an attempt to simplify and improve risk stratification, researchers have focused their attention on the development and validation of various risk scores over the past decade. The in-hospital GRACE risk score was described in 2003 and the six-month GRACE risk score one year later.5,6 Both were validated in large external data sets, and demonstrated superiority to subjective global risk assessment for in-hospital, six-month and long-term mortality and cardiovascular events.9,20,22 D’Ascenzo et al. reported that the GRACE risk score had a better discriminatory accuracy than the TIMI risk score in predicting mortality and cardiovascular events in the short and long term, with C-statistics of 0.82 and 0.81, respectively.23 However, no study has specifically proved the utility of the GRACE risk score within 30 days post-discharge. Our study has shown the independent predictive value of the six-month GRACE risk score to predict not only early post-discharge mortality, but also 30-day cardiovascular morbidity (reinfarction, heart failure, and stroke). In contrast, the in-hospital GRACE risk score did not remain as an independent predictor of events in the early phase after discharge.
Our findings also indicate that early mortality and readmission among ACS patients in Europe are low (<3% in our study). This contrasts with reported data from the USA, where 30-day mortality and post-discharge cardiovascular readmission rates were higher (around 10%).11,13,18 Kociol et al. recently demonstrated an association between length of stay (LOS) and readmission rates, which accounted for the higher readmission rate in the USA.10 In this study, the USA had the lowest median LOS among all countries, resulting in suboptimal outcomes with higher rates of readmissions. In our study, LOS was markedly higher than in US populations, which could be explained by greater attention to certain medical problems that would result in a reduction in early mortality and cardiovascular readmission rates.
Clinical implicationsThe use of the six-month GRACE risk score is crucial in the context of increasing interest in using hospital readmission as a quality metric, linked to correct clinical practice. Although the GRACE score can categorize ACS patients into predicted risk groups for early mortality and readmission after discharge, it does not distinguish between preventable or unpreventable events. However, it can identify high-risk patients for whom there is the greatest potential for preventing early events. Future efforts should be devoted to developing methods for specifically identifying those cardiovascular events that could have been prevented through improved quality of care.24
LimitationsThese data should be interpreted in the context of this study's limitations. First, it is a retrospective analysis of clinical data from a single center. Although a multivariate model was used to adjust for potential confounders, unmeasured or residual confounding may remain. Second, we have no data regarding medication compliance or socioeconomic and educational variables, which can affect the occurrence of early events. Third, the 30-day post-discharge mortality and cardiovascular readmission rates were very low, which limits the analysis of each of the endpoints (mortality, reinfarction, heart failure, and stroke) separately.
ConclusionsThe six-month GRACE clinical risk score facilitates the identification of individual patients who are at high risk of early mortality and readmission, and is a critical step on the path to reduce early mortality and cardiovascular hospital readmission rates. Newly designed interventions that have the potential to limit preventable early events, reduce healthcare costs, and improve care may have the greatest impact on this vulnerable population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like thank all the staff members (physicians, nurses and auxiliary members) and colleagues in the cardiology department and coronary care unit for their support.