Prompt identification of higher-risk patients presenting with ST-segment elevation myocardial infarction (STEMI) is crucial to pursue a more aggressive approach.
ObjectiveWe aimed to assess whether the modified shock index (MSI), the ratio of heart rate to mean arterial pressure, could predict six-month mortality among patients admitted with STEMI.
MethodsA retrospective observational cohort study was performed in a single center including 1158 patients diagnosed with STEMI, without cardiogenic shock on admission, between July 2009 and December 2014. They were divided into two groups: group 1 – patients with MSI<0.93 (72%); group 2 – patients with MSI≥0.93 (28%). The primary endpoint was six-month all-cause mortality.
ResultsMSI≥0.93 identified patients who were more likely to have signs of heart failure (p=0.002), anemia (p<0.001), renal insufficiency (p=0.014) and left ventricular systolic dysfunction (p=0.045). They more often required inotropic support (p<0.001), intra-aortic balloon pump (p<0.001) and mechanical ventilation (p<0.001). Regarding in-hospital adverse events, they had a higher prevalence of malignant arrhythmias (p=0.01) and mechanical complications (p=0.027). MSI≥0.93 was an independent predictor of overall six-month mortality (adjusted HR 2.00, 95% CI 1.20-3.34, p=0.008).
ConclusionMSI was shown to be a valuable bedside tool which can rapidly identify high-risk STEMI patients at presentation.
A identificação precoce dos doentes (dts) de maior gravidade que se apresentam com enfarte com supradesnivelamento do segment ST (EAMCSST) é fundamental para uma abordagem mais eficaz e/ou segura.
ObjetivoAvaliar se o índice de choque modificado (ICM) – razão entre a frequência cardíaca e a pressão arterial média – poderá ser um preditor de mortalidade aos seis meses, nos doentes admitidos com enfarte com EAMCSST.
MétodosEstudo observacional, unicêntrico, retrospetivo que incluiu 1158 doentes admitidos com o diagnóstico de EAMCSST, sem choque cardiogénico à admissão, desde julho de 2009 a dezembro de 2014. Os doentes foram divididos em dois grupos: grupo 1 – dts com ICM<0,93 (72%); grupo 2 – dts com ICM≥0,93 (28%). O endpoint primário foi a ocorrência de morte por todas as causas aos seis meses.
ResultadosOs doentes com ICM≥0,93 apresentavam mais frequentemente sinais de insuficiência cardíaca (p=0,002), anemia (p<0,001), insuficiência renal (p=0,014) e disfunção ventricular esquerda (p=0,045) à admissão. Estes doentes necessitaram mais frequentemente de suporte aminérgico (p<0,001), suporte com balão intra-aórtico (p<0,001) e ventilação mecânica invasiva (p<0,001). Relativamente aos eventos hospitalares adversos, os doentes com ICM≥0,93 apresentaram mais frequentemente arritmias malignas (p=0,01) e complicações mecânicas (p=0,027). O valor de ICM≥0,93 mostrou-se um preditor independente de mortalidade por todas as causas aos seis meses – HR ajustada 2,00, 95% CI (1,20-3,34), p=0,008.
ConclusãoO índice de choque modificado mostrou ser uma ferramenta útil, capaz de estratificar rapidamente os doentes com EAMCSST de maior risco.
In daily practice, when dealing with ST-segment elevation myocardial infarction (STEMI), it is important to identify patients who may potentially suffer complications.
Risk assessment provides an opportunity to estimate the patient's prognosis, alerting the physician to possible hazards, in order to pursue a more aggressive approach.1 Several risk stratification systems have been developed, such as Thrombolysis In Myocardial Infarction (TIMI) and the Global Registry of Acute Coronary Events (GRACE), but they are time-consuming and difficult to perform routinely at the bedside.2–5 It is crucial to find an easier method to stratify STEMI patients, in order to recognize subclinical indicators of worse prognosis, such as cardiogenic shock, early.
In the GUSTO trial, cardiogenic shock was reported to occur on average 12hours after STEMI presentation in patients who were not considered to have cardiogenic shock at the time of initial assessment. Some of these patients may have had subclinical shock with no sign of organ hypoperfusion.1
The shock index – the ratio of heart rate to systolic blood pressure (SBP) – is recognized as a predictor of hemodynamic instability. It is an easy tool to assess prognosis in different settings, including STEMI.6–9 A more recent index, the modified shock index (MSI), which is the ratio of heart rate to mean arterial pressure (MAP), has been shown in small studies to predict mortality in medical and trauma emergency patients.10–12 The purpose of the present study was to assess the MSI as a predictor of six-month all-cause mortality among patients admitted with STEMI.
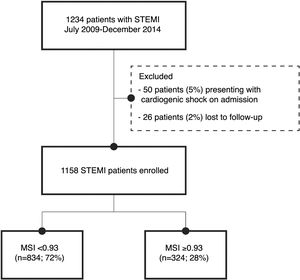
MethodsStudy populationThe study population included 1234 patients admitted with a diagnosis of STEMI between July 2009 and December 2014, either directly from the community to our center or transferred from one of its satellite hospitals, to perform emergent percutaneous coronary intervention. Of these, 26 patients were excluded as lost to follow-up (2%), and 50 patients (5%) presented with cardiogenic shock, defined as Killip class IV, on admission. Therefore, the study population consisted of 1158 patients without cardiogenic shock, presenting within 12hours of symptom onset and with persistent ST-segment elevation or new left bundle branch block, or>12hours after symptom onset and with ongoing ischemia, life-threatening arrhythmias or stuttering electrocardiogram (ECG) changes.
The diagnosis of STEMI was based on the presence of chest pain suggestive of myocardial ischemia, a 12-lead ECG showing persistent ST-segment elevation of≥2.5mm in men aged<40 years, >2mm in men aged≥40 years, and>1.5mm in women, in leads V2-V3 and/or>1mm in other leads (in the absence of left ventricular hypertrophy or left bundle branch block), or new left bundle branch block, and increased serum biomarkers of cardiac injury. The biomarkers used were cardiac troponin I and CK-MB, with a positive threshold of 0.06 and 3.5 ng/ml, respectively.
Heart failure was defined as Killip class≥2 during hospitalization.
Malignant arrhythmias were defined as ventricular fibrillation or sustained ventricular tachycardia.
Clinical data and the Modified Shock IndexDemographic, clinical, laboratory, echocardiographic and coronary angiographic data were collected prospectively and recorded in an electronic database (SIMACARDIO), in accordance with our department's protocol for patients admitted to the coronary care unit.
Regarding laboratory data, N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were measured within 24hours of admission. Estimated glomerular filtration rate (eGFR) was obtained at presentation using the abbreviated Modification of Diet in Renal Disease (MDRD) formula. Anemia was defined according to the World Health Organization criteria (hemoglobin<12g/dl in women and<13g/dl in men).
Echocardiographic data were obtained from the first echocardiogram performed within 24hours of admission or as soon as a mechanical complication was suspected. Left ventricular systolic dysfunction was defined as left ventricular ejection fraction≤40%. Right ventricular systolic dysfunction was defined as tricuspid annular plane systolic excursion<16mm.
Significant coronary artery disease on coronary angiography was defined as≥50% stenosis of the left main artery or≥70% in other coronary arteries. Severe coronary disease was defined as left main disease and/or three-vessel disease. Coronary revascularization was defined as successful percutaneous or surgical coronary intervention in order to restore blood flow.
Preprocedural systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in the catheterization laboratory with the guide catheter placed in the ascending aorta. Heart rate was obtained at the same time from the corresponding ECG. MAP was calculated using the formula ((2×DBP)+SBP)/3. Preprocedural MSI was calculated using the formula heart rate/MAP.
Follow-up and adverse eventsThe study's primary endpoint was six-month all-cause mortality and the secondary endpoint was the occurrence of cardiogenic shock during hospital stay.
Patients in this study were included in the National Registry of Acute Coronary Syndromes and were monitored for six months or until occurrence of the primary outcome. Follow-up was by phone calls and consultation of hospital records.
Statistical analysisReceiver operating characteristic (ROC) curve analysis was used to determine the optimal threshold of MSI. Categorical variables were expressed as percentages and compared by the chi-square test or Fisher's exact test. Continuous variables were tested for normal distribution by the Kolmogorov-Smirnov test; all continuous variables had a normal distribution, and so between-group differences were compared using the t test and were expressed as means±standard deviation. Binary logistic regression analysis was performed to determine the independent predictors of occurrence of cardiogenic shock during hospital stay. Only four variables with statistical significance on univariate analysis were included, given the small numbers of events in the study. Cox proportional hazards regression analysis was used to determine independent predictors of six-month all-cause mortality, including only variables with statistical significance on univariate analysis. Kaplan-Meier survival curves were constructed to compare event-free survival at six months according to the threshold value obtained for MSI. The log rank test was used to test the equality of the survival function across groups. A two-sided p<0.05 was considered statistically significant. All statistical analyses were performed with SPSS software, version 21 (IBM SPSS Inc., Chicago, IL).
ResultsThe optimal threshold for MSI was determined based on ROC curve analysis. The area under the curve (C-statistic) was 0.636 (95% confidence interval [CI]: 0.573-0.700; p<0.001). The shortest distance to the upper left corner and Youden's index were used to identify the optimal threshold, which was 0.93 (sensitivity of 65% and specificity of 73%). Patients were divided into two groups: group 1 – those with MSI<0.93 (n=843, 72%); and group 2 – those with MSI≥0.93 (n=324, 28%) (Figure 1). The groups were compared in terms of baseline characteristics, laboratory findings and adverse events.
Baseline patient characteristicsThe mean age of the study population was 61.70±13.5 years; there was no significant age difference between the groups (Table 1).
Baseline patient characteristics, clinical presentation, echocardiographic findings and coronary angiography, according to the Modified Shock Index.
| MSI<0.93 (72%; n=834) | MSI≥0.93 (28%; n=324) | p | |
|---|---|---|---|
| Demographic | |||
| Age (years), mean (SD) | 61±13 | 62±14 | 0.235 |
| Female (%) | 16.5 (138) | 21.9 (71) | 0.033 |
| Cardiovascular risk factors (%) | |||
| Diabetes | 21.6 (180) | 28.4 (92) | 0.014 |
| Hypertension | 56.6 (472) | 57.4 (186) | 0.802 |
| Dyslipidemia | 50.8 (423) | 47.8 (155) | 0.369 |
| Active smoker | 38.5 (321) | 38.9 (126) | 0.900 |
| Ex-smoker | 18.1 (150) | 15.3 (49) | 0.259 |
| BMI (kg/m2), mean±SD | 27±4 | 27±4 | 0.169 |
| Previous cardiovascular history (%) | |||
| Myocardial infarction | 9.6 (80) | 8.6 (28) | 0.618 |
| Angina | 8.8 (73) | 7.1 (23) | 0.359 |
| CABG | 1.1 (9) | 1.2 (4) | 0.822 |
| PCI | 7.0 (58) | 5.6 (18) | 0.388 |
| Stroke | 5.4 (45) | 8.0 (26) | 0.09 |
| Previous medication (%) | |||
| Aspirin | 14.6 (122) | 14.8 (48) | 0.936 |
| Beta-blockers | 16.2 (135) | 10.5 (34) | 0.359 |
| Statins | 26.6 (222) | 26.9 (87) | 0.822 |
| ACE inhibitors or ARBs | 31.9 (266) | 38.3 (124) | 0.045 |
| Diuretics | 15.8 (105) | 19 (47) | 0.205 |
| Anticoagulants | 2.1 (14) | 1.6 (4) | 0.660 |
| Clinical presentation | |||
| SBP (mmHg), mean±SDa | 135±26 | 112±20 | <0.001 |
| DBP (mmHg), mean±SDa | 83±15 | 69±13 | <0.001 |
| HR (bpm), mean±SDa | 72±14 | 92±16 | <0.001 |
| MAP (mmHg), mean±SDa | 100±18 | 83±14 | <0.001 |
| Acute heart failure (%)a | 14 (117) | 21.6 (70) | 0.002 |
| eGFR<60 ml/min/1.73 m2 (%)a | 14.3 (119) | 20.2 (65) | 0.014 |
| Anemia (%)a | 17.8 (148) | 29.2 (94) | <0.001 |
| Troponin peak level (ng/ml), mean±SD | 83±101 | 100±139 | 0.02 |
| NT-proBNP (pg/ml), mean±SD | 2228±4515 | 2816±4401 | 0.066 |
| CRP (mg/l), mean±SDa | 12.3±20.43 | 20.4±38.88 | <0.001 |
| Anterior infarction (%) | 65.9 (549) | 63 (204) | 0.346 |
| Echocardiographic findings (%) | |||
| LVEF≤40% | 39.1 (293) | 45.9 (135) | 0.045 |
| RV dysfunction | 5.1 (41) | 7.1 (22) | 0.18 |
| Coronary angiography (%) | |||
| Left main or three-vessel disease | 15.5 (129) | 18.8 (61) | 0.168 |
on admission.
ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; BMI: body mass index; CABG: coronary artery bypass grafting; CRP: C-reactive protein; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; HR: heart rate; LVEF: left ventricular ejection fraction; MAP: mean arterial pressure; MI: myocardial infarction; NT-proBNP: N-terminal pro-brain natriuretic peptide; PCI: percutaneous coronary intervention; RV: right ventricular; SBP: systolic blood pressure.
The group with MSI≥0.93 had a higher proportion of women (21.9% vs. 16.5%; p=0.033) and patients with diabetes (28.4% vs. 21.6%; p=0.014). There were no statistically significant differences between groups regarding other conventional cardiovascular risk factors such as hypertension, dyslipidemia or smoking, or regarding previous cardiovascular history.
On admission, patients with MSI≥0.93 more frequently presented signs of acute heart failure (21.6% vs. 14%; p=0.002), anemia (29.2% vs. 17.8%; p<0.001) and renal insufficiency (eGFR<60ml/min/1.73 m2) (20.2% vs. 14.3%; p<0.001). On echocardiographic assessment, almost half of patients with MSI≥0.93 had left ventricular dysfunction (45.9% vs. 39.1%; p=0.045).
Treatment and in-hospital proceduresThere were no statistical differences in terms of antithrombotic therapy, but patients with MSI≥0.93 were less often treated with beta-blockers (84% vs. 90%; p=0.004), angiotensin-converting enzyme (ACE) inhibitors (83% vs. 90%; p=0.001) and nitrates (14.2% vs. 19.5%, p=0.037) (Table 2). On the other hand, they more often required inotropic support (12.4% vs. 4.1%; p<0.001), levosimendan (2.8% vs. 0.4%; p=0.002) and diuretics (40.1% vs. 29.2%; p=0.002) (Table 2).
In-hospital treatment and procedures and in-hospital adverse events, according to the Modified Shock Index.
| MSI<0.93 (72%; n=834) | MSI≥0.93 (28%; n=324) | p | |
|---|---|---|---|
| In-hospital medication (%) | |||
| Aspirin | 100 (834) | 100 (324) | 1.00 |
| Clopidogrel | 100 (834) | 100 (324) | 1.00 |
| Beta-blockers | 90.1 (750) | 84.0 (272) | 0.004 |
| ACE inhibitors | 90 (749) | 83 (269) | 0.001 |
| Statins | 99 (824) | 98.1 (318) | 0.214 |
| UHF | 72.8 (606) | 78.4 (254) | 0.061 |
| LMWH | 31.9 (265) | 30.2 (98) | 0.581 |
| Nitrates | 19.5 (162) | 14.2 (46) | 0.037 |
| GP IIb/IIIa inhibitors | 18.3 (152) | 24.7 (80) | 0.015 |
| Inotropics | 4.1 (34) | 12.4 (40) | <0.001 |
| Diuretics | 29.2 (199) | 40.1 (101) | 0.002 |
| Procedures (%) | |||
| Symptom-to-balloon time (min) | 336±578 | 373±636 | 0.375 |
| FMC-to-balloon time (min) | 151±120 | 166±132 | 0.08 |
| Revascularization | 96.9 (808) | 97.7 (316) | 0.548 |
| CABG | 7.1 (46) | 6.7 (16) | 0.847 |
| Intra-aortic balloon pump | 1.3 (9) | 7.9 (20) | <0.001 |
| Non-invasive ventilation | 1.1 (7) | 6.9 (16) | <0.001 |
| Mechanical ventilation | 1.8 (11) | 5.2 (12) | <0.001 |
| In-hospital events (%) | |||
| New-onset heart failure | 24.6 (205) | 36.1 (117) | <0.001 |
| Cardiogenic shock | 4.4 (20) | 6.5 (21) | 0.001 |
| Angina after MI | 3.5 (29) | 2.5 (8) | 0.381 |
| Reinfarction | 1.9 (16) | 1.2 (4) | 0.423 |
| Acute stent thrombosis | 1.2 (10) | 0.6 (2) | 0.526 |
| Mechanical complications | 0.9 (16) | 2.8 (7) | 0.027 |
| Malignant arrhythmias | 6.1 (51) | 10.2 (33) | 0.017 |
| New-onset atrial fibrillation | 8.4 (70) | 14.2 (46) | 0.003 |
| High grade heart block | 7.0 (58) | 4.9 (16) | 0.206 |
| Respiratory tract infection | 3.2 (27) | 8.6 (28) | <0.001 |
| Stroke | 0.8 (7) | 1.5 (5) | 0.289 |
| Mortality | 2.4 (20) | 5.2 (17) | 0.013 |
ACE: angiotensin-converting enzyme; CABG: coronary artery bypass grafting; FMC: first medical contact; GP IIb/IIIa: glycoprotein IIb/IIIa; LMWH: low-molecular-weight heparin; MI: myocardial infarction; UHF: unfractionated heparin.
Total ischemic time and first medical contact-to-balloon time were not statistically different between groups. Regarding revascularization, there were no significant differences between groups, but patients with MSI≥0.93 more frequently required glycoprotein IIb/IIIa inhibitors (24% vs. 18.3%; p=0.015) due to evidence of massive thrombus during the angiographic procedure and no-reflow or slow flow situations.
Intra-aortic balloon pump support (7.9% vs. 1.3%; p<0.001) and non-invasive (6.9% vs.1.1%; p<0.001) and mechanical ventilation (5.2% vs. 1.8%; p<0.001) were used more frequently in patients with MSI≥0.93.
In-hospital adverse eventsIn the study population, 27.8% (n=322) of patients developed acute heart failure and 3.5% (n=41) developed cardiogenic shock. The proportion of patients with acute heart failure (36.1% vs. 24.6%; p<0.001) and cardiogenic shock (6.5% vs. 2.4%; p=0.001) was higher in patients with MSI≥0.93.
Binary logistic regression analysis was performed for development of cardiogenic shock during hospital stay using the previously identified predictors. Right ventricular dysfunction (adjusted odds ratio [OR] 5.0, 95% CI 2.05-12.21; p<0.001) and left ventricular dysfunction (adjusted OR 4.87, 95% CI 1.12-4.78; p=0.001) were the strongest independent predictors, although the presence of acute heart failure on admission (adjusted OR 3.41, 95% CI 1.63-7.16; p<0.001) and MSI≥0.93 (adjusted OR 2.731, 95% CI 1.12-4.78; p=0.023) provided additional information.
Patients with MSI≥0.93 had more episodes of malignant arrhythmias (10.2% vs. 6.1%; p=0.017), new-onset atrial fibrillation (14.2% vs. 8.4%; p=0.003), mechanical complications (2.8% vs. 0.9%; p=0.027) and respiratory tract infections (8.6% vs. 3.2%; p<0.001).
Adverse outcomesOf the total population, 3.2% (n=37) died during hospitalization, and six-month all-cause mortality was recorded in 7.2% (n=88) of patients.
Patients with MSI≥0.93 had a higher proportion of in-hospital mortality (5.2% vs. 2.4%; p=0.013; OR 2.25; 95% CI 1.17-4.36; p=0.016). Due to the low rate of in-hospital mortality it was not possible to calculate independent predictors of in-hospital mortality in this sample.
Predictors of longer-term mortalityPatients with MSI≥0.93 had also higher six-month all-cause mortality (13.3% vs. 5.4%; p<0.001); OR 2.68; 95% CI (1.73 – 4.16); p<0.001).
Table 3 shows the results of Cox proportional hazards regression analysis for six-month all-cause mortality. After adjusting for different baseline characteristics and possible confounding factors, MSI≥0.93 remained as an independent predictor (adjusted hazard ratio [HR] 2.00; 95% CI 1.20-3.34; p=0.008).
Cox proportional hazards regression analysis for six-month all-cause mortality.
| Variable | HR (95% CI) | p |
|---|---|---|
| Age | 1.06 (1.04-1.09) | <0.001 |
| eGFR<60 ml/min/1.73 m2 | 3.71 (2.11-6.55) | <0.001 |
| Acute heart failure | 1.29 (0.74-2.26) | 0.376 |
| MSI≥0.93 | 2.00 (1.20-3.34) | 0.008 |
| RV systolic dysfunction | 1.13 (0.54-2.35) | 0.746 |
| LV systolic dysfunction (LVEF<40%) | 2.55 (1.45-4.45) | 0.001 |
| Anemia | 1.52 (0.87-2.61) | 0.145 |
| Gender | 1.18 (0.66-2.10) | 0.890 |
CI: confidence interval; eGFR: estimated glomerular filtration rate by the MDRD formula; HR: hazard ratio; LV: left ventricular; LVEF: left ventricular ejection fraction; MSI: Modified Shock Index; RV: right ventricular.
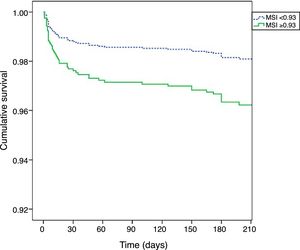
Kaplan-Meier curves (Figure 2) show that patients with MSI≥0.93 had higher mortality early after hospital admission, but their worse prognosis remained throughout the follow-up period (log rank p<0.001).
DiscussionOur study presents data on all-comers who met the criteria for STEMI, irrespective of age and comorbidities. The study reflects modern primary percutaneous coronary intervention (PCI) practice and modern care procedures, which enabled short door-to-balloon and total ischemic times, as well as the use of GP IIb/IIIa inhibitors and drug-eluting stents.
MSI is an easily accessible index that does not depend on subjective information, previous patient history or blood tests; it only depends on invasive measures of blood pressure and heart rate at the beginning of the primary PCI procedure, which are less susceptible to fill-in errors.
MSI has been demonstrated to be a valid prognostic tool in medical or trauma patients admitted to the emergency department. Liu et al. showed that an MSI of≥1.3 was associated with increased probability of intensive care unit admission or death.10 Other studies have compared the standard shock index with MSI for predicting prognosis in emergency patients and showed that MSI is a better predictor of mortality in this setting.11,12
The MSI has been tested in STEMI patients as well as in emergency patients. Our results are in line with Shangguan et al.,13 who found higher rates of all-cause mortality and major adverse cardiac events at seven days in STEMI patients who presented MSI≥1.4. They compared the shock index with MSI and concluded that the latter better predicted prognosis: MSI≥1.4 predicted higher rates for all-cause mortality (20.4% vs. 13.9%) and major adverse cardiac events (44.9% vs. 36.1%) than SI≥0.7. As the calculation of MSI uses MAP, its greater predictive power in STEMI patients is logical, since it more accurately reflects myocardial perfusion and systemic vascular resistance.13 The cut-off used by Shangguan et al. was determined on the basis of ROC curve analysis, as in our study. A possible explanation for their higher cut-off is that they did not exclude patients admitted in cardiogenic shock. Such patients are known to have higher heart rate, which can increase the cut-off obtained. Excluding patients in cardiogenic shock on admission may have led us to use a lower cut-off value, but it also helped to determine whether MSI could identify patients in the early phase of decompensation, at a stage when cardiogenic shock is not yet established.
In our study, the MSI was shown to be valuable in identifying more critical and morbid patients presenting at a pre-shock stage. Those with higher MSI more often had diabetes and on admission more often presented anemia, renal insufficiency and acute heart failure.
By identifying patients with worse prognosis, this index can enable closer monitoring and increase alertness for possible complications. In the present study, patients with higher MSI had a higher prevalence of malignant arrhythmias, mechanical complications, and respiratory tract infections. It was also a strong independent predictor of cardiogenic shock during hospital stay (OR 2.73, 95% CI 1.12-4.78; p=0.001).
In the early management of high-risk patients with relative hypotension and tachycardia, this tool can be used not only to assess risk but also to prevent iatrogenic cardiogenic shock by avoiding certain therapies, such as beta-blockers or ACE inhibitors.14
Interestingly, in our study MSI was also an independent predictor of six-month mortality in STEMI patients on multivariate analysis. One possible explanation for this is that a high MSI may identify more frail patients with comorbidities that in themselves impart a worse prognosis, such as female gender, renal insufficiency, anemia, and left ventricular systolic dysfunction. Another is its association with in-hospital adverse events such as mechanical complications, new-onset heart failure, cardiogenic shock and respiratory tract infections, which also increase frailty. A third possible explanation is the inherent hemodynamic profile of this patient group, which may hamper the introduction or titration of treatments that could modify prognosis.
Although various systems have been applied for risk stratification in STEMI patients, including the TIMI and GRACE scores, the complex and lengthy calculations involved usually make them impractical in daily clinical practice. The MSI is a valuable prognostic tool, based only on patients’ hemodynamic profile assessed on admission, that has the advantage of being calculated rapidly. MSI may be used in addition to conventional risk scores to complement risk assessment, helping physicians to implement different strategies in this population in order to change their outcomes, such as providing hemodynamic support and introducing well-timed treatments that could modify prognosis.
Study limitationsFirstly, although our patients were included in a prospective registry, this was a retrospective, non-randomized, observational study conducted in a single center, and so the results may have been influenced by identified or unidentified confounding factors. Secondly, most variables were determined by consulting medical records, which may have been incomplete. Finally, as the study's primary endpoint was all-cause mortality, this may have included not only cardiovascular death, but also death from other causes, which could bias our findings.
ConclusionIn summary, MSI≥0.93 was an independent predictor of six-month mortality. MSI is an easily accessible tool that can be used to stratify STEMI patients and guide clinical management. Nevertheless, it needs to be externally validated and compared to existing validated indices.
Conflicts of interestsThe authors have no conflicts of interest to declare.