Ischemic mitral regurgitation (IMR) is associated with increased mortality. Even after coronary artery bypass grafting (CABG), IMR reduces survival. Several studies have shown increased perioperative mortality for mitral valve replacement (MVR) in this situation, but the subject remains controversial.
ObjectiveTo investigate the impact of MVR on immediate outcomes in patients with moderate-to-severe IMR undergoing concomitant CABG compared with those undergoing CABG only.
MethodsWe performed a retrospective study of 42 patients undergoing CABG+MVR (n=16) or CABG only (n=26) at the Division of Cardiovascular Surgery of PROCAPE, between May 2007 and April 2010. Preoperative clinical characteristics, procedural characteristics, major and minor complications after surgery, preoperative and postoperative left ventricular ejection fraction (LVEF) by echocardiography, and outcome (survivor or death) were assessed.
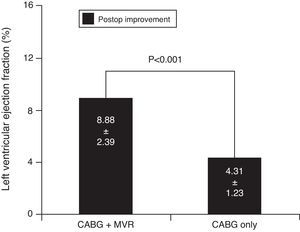
ResultsMean patient age was 63.4±8.5 years, and 64.8% (n=23) were male. The CABG+MVR group showed lower rates of postoperative low cardiac output (6.3% vs. 42.3%, p=0.014) and atrial fibrillation (6.3% vs. 38.5%, p=0.021). Both groups had higher mean LVEF in the postoperative compared with the preoperative period, but the average gain in LVEF in the CABG+MVR group was higher than in the CABG-only group (8.88±2.39 vs. 4.31±1.23, p<0.001). There was no significant difference in operative mortality (6.3% vs. 7.7%, p=0.679).
ConclusionsCABG+MVR can be performed safely in patients with moderate-to-severe IMR. CABG+MVR resulted in lower rates of complications than CABG only. Both surgical approaches resulted in significant improvement of postoperative LVEF. However, there was greater improvement in the CABG+MVR group.
A Regurgitação mitral isquémica (RMI) está associada ao aumento da mortalidade. Mesmo após a cirurgia de revascularização miocárdica (CRM), reduz a sobrevida. Vários estudos enfatizam o aumento da mortalidade perioperatória com troca valvar mitral (TVM) nesta situação, mas isto ainda é controverso.
ObjetivoInvestigar o impacto da TVM nos resultados imediatos em pacientes com RMI moderada a grave submetidos à CRM em comparação com aqueles submetidos à CRM apenas.
MétodosEstudo retrospetivo de 42 pacientes submetidos à CRM+TVM (n=16) ou CRM isolada (n=26) na Divisão de Cirurgia Cardiovascular do PROCAPE, de maio de 2007 a abril de 2010. Foram avaliadas características clínicas pré-operatórias, características do procedimento, complicações após a cirurgia, fração de ejeção do ventrículo esquerdo (FEVE) pré e pós-operatório pelo ecocardiograma e evolução (sobrevivência ou óbito).
ResultadosA idade média dos pacientes foi de 63,4 anos (±8,5), sendo 64,8% (n=23) do sexo masculino. O grupo CRM+TVM apresentou menores taxas de baixo débito cardíaco no pós-operatório (6,3 versus 42,3%, p=0,014) e fibrilação atrial (6,3% versus 38,5%, p=0,021). Ambos os grupos apresentaram maior média de FEVE no pós-operatório em comparação com o período pré-operatório; no entanto, o ganho médio da FEVE no grupo CRM+TVM foi maior em comparação com o grupo CRM isolada (8,88±2,39 versus 4,31±1,23, p<0,001). Não houve diferença significativa nas taxas de mortalidade operatória (6,3 versus 7,7%, p=0,679).
ConclusõesCRM+TVM pode ser realizada com segurança em pacientes que têm RMI moderada a grave. CRM+TVM resultou em menores taxas de complicações do que a CRM isolada. Ambas as abordagens cirúrgicas resultaram em melhoria significativa da FEVE pós-operatória, por outro lado, houve maior ganho no grupo CRM+TVM.
Ischemic mitral regurgitation (IMR) is defined as mitral valve (MV) insufficiency precipitated by myocardial infarction, with normal leaflet and chordal morphology. IMR usually occurs with right or circumflex coronary infarction involving the posterior ventricular wall, posterior papillary muscle, and adjacent mitral annulus.1 Common anatomic features include annular dilatation, displacement of papillary muscles, and varying degrees of leaflet restriction or tethering.1 There is a clear association between IMR and increased late mortality.2–7 Even after revascularization, IMR reduces late survival.2–7
Several studies have shown increased perioperative mortality for valve replacement in this situation, but the subject remains controversial.8–12
The objective of this study was to investigate the differences in immediate postoperative results (in-hospital complications and evolution) in patients with IMR undergoing coronary artery bypass grafting (CABG) with intraoperative correction of valve dysfunction by mitral valve replacement (MVR) compared with those undergoing CABG only.
MethodsStudy populationWe studied 42 consecutive patients with coronary artery disease associated with moderate-to-severe IMR undergoing CABG at the Division of Cardiovascular Surgery of Pronto Socorro Cardiológico de Pernambuco (PROCAPE), between May 2007 and April 2010.
Definition of ischemic mitral regurgitationIMR was defined as valve insufficiency caused by coronary artery disease. All patients had a history of myocardial infarction, ejection fraction <50% by echocardiography and moderate-to-severe functional MVR (without intrinsic changes in valve leaflets and/or subvalvular apparatus). All patients had one or more left ventricular (LV) segmental wall motion abnormalities and significant coronary disease in the territory supplying the wall motion abnormality.
Exclusion criteriaPatients with rheumatic, myxomatous, infectious, or congenital diseases of the mitral valve were excluded. Patients with mitral regurgitation due to papillary muscle rupture, torn or elongated chordae tendineae, or ballooning or scalloping of the mitral leaflets were not considered to have IMR. Patients with mild MVR were also not considered for the study.
Study designWe performed a retrospective study using medical records and initially generated two groups: CABG+MVR (biological valve prosthesis) or CABG only. In each instance, the operative approach for concomitant MVR had been chosen by the attending surgeon.
Variables and outcomesThe following variables were compared:
- (1)
Preoperative clinical characteristics: age >70 years, gender (male or female), obesity (body mass index≥30kg/m2), hypertension, diabetes, smoking, chronic obstructive pulmonary disease (dyspnea or chronic cough and prolonged use of bronchodilators or corticosteroids and/or radiological changes including opacification due to hyperinflation and/or elevation of the ribs and/or flattening of the diaphragm), renal disease (creatinine ≥2.3mg/dl or preoperative dialysis), myocardial infarction <30 days previously, EuroSCORE ≥6 (high risk), and New York Heart Association (NYHA) functional class I, II, III or IV;
- (2)
Procedural characteristics: number of aortocoronary bypasses; as all patients underwent on-pump surgery, we assessed cardiopulmonary bypass (CPB) duration (in min) and aortic clamp time (in min);
- (3)
Major procedure-related complications: hemorrhagic shock, neurologic complications (stroke or transient ischemic attack), low cardiac output (signs of poor peripheral and/or central perfusion including cold extremities, oliguria/anuria or decreased level of consciousness, and need for inotropic support with dopamine 4μg/kg/min for at least 12hours or intra-aortic balloon pump to maintain systolic blood pressure greater than 90mmHg), and renal complications (creatinine ≥2.3mg/dl or postoperative dialysis);
- (4)
Minor procedure-related complications: respiratory complications (pulmonary infection, acute respiratory distress syndrome, atelectasis, need for intubation for more than 48hours), atrial fibrillation (AF) after surgery, need for multiple transfusions (more than three units of packed red blood cells);
- (5)
Preoperative and postoperative left ventricular ejection fraction (LVEF) by echocardiography performed during hospitalization;
- (6)
Length of stay in intensive care unit (days) and hospital (days);
- (7)
Outcome (survivor or death).
Statistical analysis and interpretation were performed in SPSS (Statistical Package for the Social Sciences) version 15. Data were stored in duplicate to validate the consistency of the data and the analysis in order to minimize error.
Univariate analysis for categorical variables was performed with the chi-square test or Fisher's exact test, as appropriate. The Student's t test was used for continuous variables. Verification of the hypothesis of equality of variances was performed using Levene's F test. Values of p<0.05 were considered statistically significant.
Ethical considerationsThis study was approved by the Research Ethics Committee of Complexo Hospitalar do Hospital Universitário Oswaldo Cruz/Pronto Socorro Cardiológico de Pernambuco – HUOC/PROCAPE, file no. 132/2009.
ResultsPopulation characteristicsMean patient age was 63.4±8.5 years; 64.8% (n=23) were male and 35.2% (n=19) female.
The 42 patients with moderate-to-severe IMR were among 542 patients who underwent CABG consecutively during the study period, and thus the prevalence of moderate-to-severe IMR was 8.1% among operated patients. Of these, 38.1% (n=16) underwent CABG+MVR and 61.9% (n=26) underwent CABG only.
For almost all the preoperative variables (Table 1), there were no statistically significant differences, except for the proportion of patients in NYHA class III/IV, with more patients in this class in the CABG+MVR group than in the CABG-only group (68.7% vs. 30.8%, p=0.012).
Characteristics of patients undergoing coronary artery bypass grafting with and without mitral valve replacement.
| Variable | CABG+MVR | CABG | Total | p | |||
| n | % | n | % | n | % | ||
| Age >70 years | 5 | 31.3 | 8 | 30.8 | 13 | 31.0 | 1.000b |
| Male gender | 9 | 56.3 | 14 | 53.8 | 23 | 64.8 | 0.879b |
| Obesity | 1 | 6.3 | 3 | 11.5 | 4 | 9.5 | 1.000a |
| Hypertension | 15 | 93.8 | 22 | 84.6 | 37 | 88.1 | 0.633a |
| Diabetes | 4 | 25.0 | 11 | 42.3 | 15 | 35.7 | 0.256b |
| Smoking | 7 | 43.8 | 8 | 30.8 | 15 | 35.7 | 0.394b |
| COPD | 2 | 12.5 | 0 | 0.0 | 2 | 4.8 | 0.139a |
| Renal disease | 3 | 18.8 | 0 | 0.0 | 3 | 7.1 | 0.320a |
| MI <30 days | 8 | 50.0 | 12 | 46.2 | 20 | 47.6 | 0.808b |
| EuroSCORE ≥6 | 9 | 56.3 | 11 | 42.3 | 20 | 47.6 | 0.380b |
| NYHA class | 0.012a | ||||||
| I/II | 5 | 31.3 | 18 | 69.2 | 23 | 54.8 | |
| III/IV | 11 | 68.7 | 8 | 30.8 | 19 | 45.2 | |
As expected (Table 2), patients undergoing CABG+MVR showed a higher proportion with prolonged CPB time (p=0.001) and prolonged aortic clamp time (p<0.001). There was no difference in the proportion of the number of coronary bypasses as a categorical variable; however, when analyzed as a continuous variable, the CABG+MVR group underwent more coronary bypasses than the CABG-only group (3.06±0.85 vs. 2.46±0.81, p=0.028).
Characteristics of coronary artery bypass grafting procedures with and without mitral valve replacement.
| Variable | CABG+MVR | CABG | Total | p | |||
| n | % | n | % | n | % | ||
| Number of bypasses | 0.058b | ||||||
| 1 | 0 | 0.0 | 1 | 3.8 | 1 | 2.4 | |
| 2 | 4 | 25.0 | 15 | 57.7 | 19 | 45.2 | |
| 3 or more | 12 | 75.0 | 10 | 38.5 | 22 | 52.4 | |
| CPB time (min) | 0.001b | ||||||
| ≤90 | 0 | 0.0 | 11 | 42.3 | 11 | 26.1 | |
| 91–120 | 4 | 25.0 | 8 | 30.8 | 12 | 28.5 | |
| >120 | 12 | 75.0 | 7 | 27.9 | 19 | 45.2 | |
| Aortic clamp time (min) | <0.001a | ||||||
| ≤60 | 0 | 0.0 | 14 | 53.8 | 14 | 30.4 | |
| 61–90 | 7 | 43.7 | 12 | 46.2 | 19 | 45.2 | |
| >90 | 9 | 56.3 | 0 | 0.0 | 9 | 24.4 | |
The CABG+MVR group showed lower rates of low cardiac output (6.3% vs. 42.3%, p=0.014) and atrial fibrillation (6.3% vs. 38.5%, p=0.021). The other variables showed no statistically significant differences (Table 3).
Complications and mortality in patients undergoing coronary artery bypass grafting with and without mitral valve replacement.
| Variable | CABG+MVR | CABG | p | OR (CI 95%) | ||
| n | % | n | % | |||
| Shock/hemorrhage | 0 | 0.0 | 0 | 0.0 | – | – |
| Low cardiac output | 1 | 6.3 | 11 | 42.3 | 0.014b | 0.09 (0.01–0.81) |
| Renal complications | 0 | 0.0 | 5 | 19.2 | 0.138b | 0.11 (0.01–1.68) |
| Neurologic complications | 2 | 12.5 | 3 | 11.5 | 1.000b | 1.10 (0.08–10.82) |
| Atrial fibrillation | 1 | 6.3 | 10 | 38.5 | 0.021b | 0.11 (0.01–0.96) |
| Respiratory complications | 3 | 18.8 | 9 | 34.6 | 0.316b | 0.44 (0.06–2.28) |
| Multiple transfusion | 3 | 18.8 | 11 | 42.3 | 0.116a | 0.31 (0.05–1.64) |
| Death | 1 | 6.3 | 2 | 7.7 | 0.679b | 0.80 (0.03–12.98) |
There were no statistically significant differences between groups in length of stay in intensive care (7.81±5.86 vs. 8.35±7.10 days, p=0.802) or in hospital (44.06±18.61 vs. 40.54±19.66 days, p=0.568).
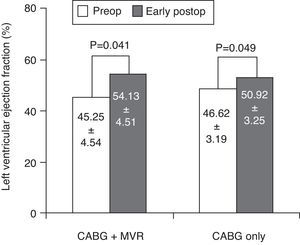
Assessment of left ventricular functionBoth the CABG+MVR group (54.13±4.51 vs. 45.25±4.54, p=0.041) and the CABG-only group (50.92±3.25 vs. 46.62±3.19, p=0.049) had higher mean LVEF in the postoperative period than in the preoperative period (Figure 1).
Both groups thus had higher mean LVEF in the postoperative than in the preoperative period, showing an improvement in LV function after surgical procedures. However, the average gain in LVEF in the CABG+MVR group was higher than that in the CABG-only group (8.88±2.39 vs. 4.31±1.23, p<0.001) (Figure 2).
MortalityThere was no significant difference between groups in operative mortality (6.3% vs. 7.7%, p=0.679) (Table 3).
DiscussionThe prevalence of moderate-to-severe IMR detected by transthoracic echocardiography and/or cardiac catheterization in myocardial infarction patients with coronary artery disease ranges from 3% to 12%.13,14 The prevalence of 8.1% observed in our study is within the range reported in the literature.
Mitral regurgitation causes atrial hemodynamic overload, which leads to tissue fibrosis; consequently, a non-homogenous distribution of diastolic depolarization potentials, refractory periods, and conduction properties occurs within the atrial muscle.15 All of these factors enhance the probability of reentry circuits forming around areas with longer refractory periods.15 MVR eliminates regurgitation, thereby reducing atrial hemodynamic overload and interrupting the cascade of events that culminates in the development of postoperative atrial fibrillation. In our study, the CABG+MVR group, despite longer exposure to CPB (known as a risk factor for developing postoperative AF16) showed a lower rate of AF than the CABG-only group (6.3% vs. 38.5%, p=0.021).
It seems logical to assume that the volume overload associated with mitral regurgitation will be particularly detrimental to patients with compromised LV function.17 There is also a loss of flow to the aorta, since part of the ejected volume is directed to the left atrium. MVR eliminates the volume overload and the loss of volume ejected toward the left atrium, displacing the entire cardiac output in the correct direction (to the aorta). This explains the lower rate of low cardiac output in the CABG+MVR group (despite longer exposure to CPB, known as a risk factor for development of postoperative low cardiac output16) compared to CABG only (6.3% vs. 42.3%; p=0.014).
Segmental wall motion abnormalities and LV distortion and remodeling after myocardial infarction displace the papillary muscles from the mitral annulus.18 This displacement puts excessive tension on the chordae, resulting in apical mitral leaflet tethering, restricting coaptation during systole.19–21 Leaflet tethering is compounded by LV contractile dysfunction, which decreases the closing force on the leaflets.21 Once IMR begins, end-diastolic LV volume and wall stress increase in tandem with preload.21 LV mass also increases progressively without a concomitant increase in end-diastolic wall thickness, resulting in generalized loss of myocardial contractile function.22 Increased wall stress causes further LV dysfunction,22 which in turn results in further papillary muscle displacement and leaflet tenting. If LV dilation occurs, it leads to annular enlargement and dysfunction, thereby increasing valvular incompetence.23 Chronic IMR therefore begets MR in a self-perpetuating manner. CABG surgery may interrupt the perpetuation and/or progression of this vicious cycle.
MVR is still a reasonable surgical option in many patients with IMR, mainly because of its reliability and reproducibility. It should be considered for patients with acute IMR, and for those with chronic IMR and multiple comorbidities, complex regurgitant jets (noncentral or more than one jet), or severe tethering of both MV leaflets.9,24,25
Some studies indicate greater perioperative mortality associated with this procedure, suggesting that preference should be given to less aggressive procedures such as CABG only or CABG associated with MVR.8,9 However, in our study, although the CABG+MVR group had a statistically significant higher prevalence of patients with worse functional class, there was no difference in operative mortality (6.3% vs. 7.7%; p=0.679) between groups. A possible explanation for this is that, unlike in other studies, MVR was the first choice for mitral valve surgery, and not a result of initial unsuccessful mitral valve repair (reoperation and/or prolonged CPB time as a function of failed valve repair might have increased operative mortality in this group). In addition, surgical intervention can prevent the LV overload and remodeling that results from mitral regurgitation, which may improve surgical outcomes.25
Goland et al.26 studied changes in LVEF in 83 patients with moderate IMR who underwent CABG associated with MVR (n=28) or CABG only (n=55). Patients who underwent CABG only showed significant improvement in LVEF in the early postoperative period (39±11 vs. 45±13; p=0.002), as did the CABG+MVR group (37±11 vs. 44±11; p=0.02). We also observed the same results of MVR, with the CABG+MVR group showing higher mean LVEF in the postoperative than in the preoperative period (54.13±4.51 vs. 45.25±4.54, p=0.041), as did the CABG-only group (52.92±4.25 vs. 48.62±4.19, p=0.049). So we observed improvement in postoperative LVEF in both groups. Furthermore, we found that the average gain in LVEF in the CABG+MVR group was higher than in the CABG-only group (8.88±2.39 vs. 4.31±1.23, p<0.001). As can be seen, in the case of LVEF, CABG+MVR provides better improvement than CABG only.
Although we did not analyze the approach to mitral valve repair, some considerations should be borne in mind. This is a technique intended to reduce or eliminate mitral regurgitation, while preserving the valve. Several investigators have suggested that repair is better than replacement for patients with IMR.11 Others, however, have documented similar survival after repair and after replacement.27 Late survival is poor for all approaches, with most patients dying within seven years of surgery.9 However, choice of surgical procedure has an important impact on survival. Among the most severely ill patients, the survival benefit of mitral valve surgery (by either valve repair or replacement) is diminished, which leads us to conclude that clinical status is an important determinant of survival.9 It seems that the “repair vs. replacement” debate remains undecided, although there is a strong tendency in the medical community in favor of repair.
Study limitationsThe chief limitation of this study is its retrospective nature, with various sources of bias. Selection bias and lack of a uniform surgical experience (different surgeons operating) are important limitations. In many cases the surgeon did not opt for valve repair because of the unavailability of intraoperative transesophageal echocardiography at our institution. Decisions to perform concomitant MVR were made on the basis of surgical considerations and preferences. The surgeons may have selected replacement for patients who had worse heart failure, and thus may have replaced valves in the more severe or symptomatic patients. A randomized prospective design would overcome this limitation.
Another limitation is the lack of uniformity of echocardiographic evaluation of mitral regurgitation grade and complete follow-up, since the echocardiograms were performed by several operators using different equipment and our results are restricted to the in-hospital period.
An important limitation is that ventricular diameters (systolic and diastolic) were not taken into consideration, which may well have influenced the results to some extent.
Small sample size is another limitation of this study. This is the consequence of selecting a very homogeneous group with only moderate-to-severe mitral regurgitation, a history of myocardial infarction, impaired LV function, and a single mitral valve surgical approach (valve replacement). This prevented the application of multivariate logistic regression analysis, so the study is limited to the use of univariate analysis, which may affect the consistency of the presented evidence.
ConclusionTaking into account the severity of this population, patients who underwent CABG only or CABG+MVR surgery experienced no statistically different mortality rates, despite the presence of multiple comorbidities and impaired LVEF. MVR can be performed safely, concomitantly with CABG, in patients with moderate-to-severe IMR. In such patients, the combined procedure resulted in lower rates of postoperative atrial fibrillation and low cardiac output than CABG only. Both surgical approaches resulted in significant improvement in postoperative LVEF. However, there was greater improvement in the combined surgery group, which may result in greater benefit to this group. Despite being a more aggressive approach, the combined surgical procedure did not increase morbidity or mortality.
Conflicts of interestThe authors have no conflicts of interest to declare.