Cardiovascular disease (CVD) and cancer are the leading causes of death in developed countries. Improvements in early detection and major advances in cancer treatments have led to a growing number of survivors. However, cancer patients, including survivors, have a high risk for CVD that can be explained by multiple factors, including toxicity of cancer treatments (old and new drugs, chest radiotherapy), shared cardiovascular risk factors like age, genetics, obesity, smoking and lifestyle, and older age with higher burden of pre-existing CVD. Coronary artery disease is a frequent comorbidity in patients being treated for cancer and in survivors. Patients with cancer are unique in that they are a diverse group of patients who are at increased risk of both bleeding and ischemic complications, depending on the type and stage of cancer, and require a highly individualized approach.1 Malignancies themselves are heterogeneous in terms of their prognosis and adverse systemic effects, and their effects on percutaneous coronary intervention (PCI) outcomes are not identical. Moreover, there are limited data assessing the incidence and outcomes of acute coronary syndromes (ACS) in patients with cancer, since they are routinely excluded from clinical trials of ACS therapies, particularly those undergoing active cancer treatment.
In 2016, the Society for Cardiovascular Angiography and Interventions (SCAI), in collaboration with several other national groups, published an expert consensus document that includes advice on interventional procedures in patients with concomitant cancer and CVD. The SCAI recommends consideration of percutaneous revascularization even in cancer patients with an expected survival of less than one year, and also recommends specific strategies to address the risk of PCI-related complications in cardio-oncology patients, including the use of balloon angioplasty over stenting in patients with platelets <30 000/ml, bare-metal stents in patients with an urgent need for surgery or chemotherapy (within four weeks), and a radial approach and micropuncture techniques for vascular access, as well as the use of intracoronary imaging (intravascular ultrasound or optical coherence tomography) to ensure optimal stent apposition.
Research has been slow to fill the voids identified in this document.2 Recently, however, a few studies have shed light on some of these issues, for instance providing some reassurance that stents do not pose additional risks. In fact, the real risk is bleeding and not stent thrombosis or ischemic complications, especially with certain cancer types.
In their study published in this issue of the Journal, Mano et al.3 analyzed patients included between October 2010 and September 2019 in the Portuguese Registry of Acute Coronary Syndromes (ProACS), grouping the study population according to the presence or absence of a cancer diagnosis (active or in remission).
Among the 18 845 patients examined, 5% (934) had a diagnosis of cancer. The primary (safety) endpoint was major bleeding and secondary endpoints assessed the efficacy of ACS treatment. Patients with cancer tended to be older and had more comorbidities, major bleeding, and in-hospital mortality. When PCI was performed, there were no significant differences between the groups. Not surprisingly, cancer patients had a higher rate of bare-metal stent implantation. After propensity score matching (350 patients), there were no statistically significant differences in endpoints between the populations. The authors concluded that bleeding risk was not significantly higher in the cancer population and that accordingly cancer patients should not be excluded from state-of-the-art ACS treatment.
In the largest registry published on acute myocardial infarction (AMI) in cancer patients,4 data on 6 563 255 patients presenting with an AMI between 2004 and 2014 from the US National Inpatient Sample database were analyzed. A total of 5 966 955 had no cancer, 186 604 had current cancer, and 409 697 had a historical diagnosis of cancer.
The most common types of cancer associated with ACS were lung, prostate, and breast cancer. The study showed that survival and clinical outcomes varied significantly according to the type of cancer and metastasis status. Cancer patients had a higher bleeding risk; in particular, colon cancer presented the highest risk of major bleeding. Lung cancer was associated with the highest rates of in-hospital mortality and major adverse cardiovascular and cerebrovascular complications.
In another study from the same group, Mohamed et al.5 looked at the rates of invasive management strategies for ST-elevation myocardial infarction (STEMI) in patients with and without cancer. The authors performed a propensity score-matched analysis of a nation-wide sample of hospitalizations in the US between 2004 and 2015. Out of 1 870 815 patients with STEMI, 38 932 (2.1%) had a current cancer diagnosis. Patients with active cancers were less likely to undergo primary PCI (pPCI). Depending on cancer type, pPCI had a similar or stronger treatment effect in cancer compared to no cancer, with associated reduction of all-cause mortality and complications in both groups, and no increase in the associated risk of major bleeding.
Ueki et al.6 analyzed the Bern PCI registry (between 2009 and 2017). Among 13 647 patients, 1368 (10.0%) had an established diagnosis of cancer. After matching patients with and without a cancer diagnosis, there was no difference in a composite endpoint of device-related events, and no differences in stent thrombosis, myocardial infarction, or target lesion revascularization. However, overall deaths, cardiac death, and Bleeding Academic Research Consortium (BARC) 2 to 5 bleeding were all higher in cancer patients.7,8 Of note, these risks differed according to the time since cancer diagnosis. In patients with a recent cancer diagnosis (one year or less), the risks of cardiac death and bleeding were significantly higher, whereas no significantly increased risk was seen for either endpoint in patients whose diagnosis was 1-5 years or ≥5 years previously.
Mano et al. are to be congratulated for making an important contribution in this field by addressing the impact of cancer on ischemic and bleeding complications with ACS. However, there are some limitations that are identified by the authors, particularly that there were no data on how many patients had active cancer or cancer in remission, or on the type or stage of malignancy.
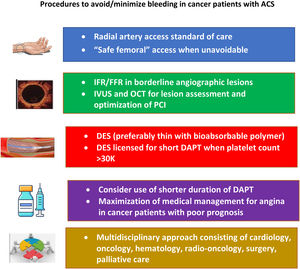
Optimal treatment of cancer patients with ACS is still unclear and subject to variability according to comorbid conditions. In cancer patients who require PCI, operators should actively pursue bleeding avoidance strategies (Figure 1). Transradial access should be the standard of care, given its lower risk of bleeding and vascular complications. The type and duration of dual antiplatelet therapy (DAPT) should consider the patient's type of cancer and the associated bleeding risk. Recent trials assessing the role of shorter-duration DAPT in patients at high bleeding risk have shown positive results in terms of decreasing bleeding without an increase in ischemic events. Newer stent platforms have also yielded promising results with shorter duration of DAPT.9,10
Procedures to avoid or minimize bleeding in cancer patients with acute coronary syndromes (adapted from Bharadwaj et al.8). DAPT: dual antiplatelet therapy; DES: drug-eluting stent; FFR: fractional flow reserve; IFR: instantaneous wave-free ratio; IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention.
Treatment of a cancer patient presenting with ACS is challenging and decisions should be individualized and based on a multidisciplinary team discussion.
Conflicts of interestThe author has no conflicts of interest to declare.