The aim of this study is to estimate the association of shortness of breath (SOB), fatigue and bilateral lower limb edema (LLE) – typical symptoms of HF – with quality of life (QOL) dimensions, measured by the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36).
MethodsThis cross-sectional study was conducted as part of the CAMELIA study (Cardiometabolic Renal Familial Study), which involved families covered by the Family Doctor Program (FDP) in Niteroi, Rio de Janeiro, Brazil. The study included 455 patients aged 30 and over, assessed by questionnaire, medical consultation, and blood and urine tests.
ResultsThe prevalence of symptoms was: fatigue 56.9%, SOB 22.6% and LLE 16.9%. There were independent and statistically significant associations between SOB and fatigue and all SF-36 dimensions, excepting emotional performance and SOB (p<0.10).
ConclusionThe combination of SOB and fatigue with low QOL can increase the positive predictive value for a clinical diagnosis of HF and is a possible alternative for prioritizing patients for closer investigation in a primary care setting.
O presente estudo visa estimar a associação de falta de ar (FA), fadiga (FD) e edema bilateral de membros inferiores (EMI), sintomas típicos de insuficiência cardíaca (IC), com dimensões da qualidade de vida (QV), mensuradas pelo Medical Outcomes Study 36-item Short Form Health Survey (SF-36).
MétodosTrata-se de um estudo transversal, parte do Estudo Camelia (Estudo Cardiometabólico Renal Familiar), que envolveu famílias assistidas pelo Programa Médico de Família de Niterói (PMF), Rio de Janeiro, Brasil. Foram incluídos 455 indivíduos de 30 anos ou mais, avaliados através de questionário, consulta médica e exames laboratoriais de sangue e urina.
ResultadosA prevalência de FA foi de 22,6%, de FD de 56,9% e de EMI, de 16,9%. Na análise multivariada observou-se relaçção estatisticamente significativa entre FA e FD com todas as dimensões do SF-36, à exceção de aspetos emocionais e FA cujo valor p foi menor do que 0,10.
ConclusãoA combinação de FA e FD com baixa pontuação de QV pode aumentar o valor preditivo positivo do diagnóstico clínico da IC, sendo uma alternativa possível para priorizar pacientes para investigação mais acurada em cenários em que o acesso a esses métodos é limitado.
Heart failure (HF) is a pandemic condition whose prevalence is steadily increasing as populations age.1 In Brazil, it is second only to childbirth as a cause of hospitalizations in adults and is the leading cardiovascular cause of hospitalization.2
The triad of HF symptoms (shortness of breath [SOB], fatigue and bilateral lower limb edema [LLE]) is found in more than 50% of patients, reaching 80% in some populations.3 These symptoms restrict patients’ daily life and impact their quality of life (QOL).3 A clinically based diagnosis of HF (clinical history, signs and symptoms) has low accuracy,4 and access to more accurate methods such as the B-type natriuretic peptide (BNP) blood test can be problematic in a primary care (PC) setting due to cost and limited availability of skilled professionals,4 resulting in delayed treatment. Early diagnosis and intervention can postpone the development of the disease,4 reverse left ventricular remodeling and improve QOL in several ways.5
This study aims to estimate the association of complaints of SOB, fatigue and LLE (typical symptoms of HF) with QOL in the population covered by the Family Doctor Program (FDP) in Niteroi, Rio de Janeiro, Brazil. Such an association may point to a possible alternative for prioritizing access to more accurate tests for the diagnosis of HF in patients with HF symptoms and low QOL.
MethodsThis was a cross-sectional study, conducted within the CAMELIA study, which involved families covered by the FDP of Niteroi, Rio de Janeiro, Brazil. Randomly sampled individuals (index cases) and their spouses and children between 12 and 30 years of age were invited to participate. The index cases were classified as non-diabetic hypertensive, non-hypertensive diabetic, hypertensive diabetic or non-diabetic and non-hypertensive.
In this study, individuals were classified as positive for SOB, fatigue and LLE based on positive responses to direct questions in the self-administered questionnaire. For the QOL assessment, the Medical Outcomes Study 36-item Short Form Health Survey (SF-36) was used. The SF-36 is a self-administered general QOL questionnaire consisting of 36 items that evaluate the following dimensions: functional capacity, physical aspects, pain, general health, vitality, social and emotional aspects and mental health.6 For each QOL dimension, a median score was calculated and considered as the cutoff point. To assess depression, the Beck Depression Inventory short form (BDI-SF) consisting of 13 items,7 each with four possible answers, was used. Scoring was in a continuous and dichotomous form, with a cutoff of 9/10.
Of the 1098 CAMELIA study participants, 455 individuals (41.43%) were eligible for this study. Individuals over 30 years of age (664) were included, and the study excluded those with no available information for anthropometric measurements (10), hematocrit (25), glucose (68), and total cholesterol (57) and those who did not respond to the SF-36 (41) or to questions regarding fatigue (7), SOB and LLE (one person did not respond to either the SOB or the edema questions). Missing data were considered random and were due to difficulty in keeping the participants in the clinic on days when the wait was longer due to technical difficulties in processing blood and/or urine samples. Comparison between the two samples showed a similar demographic profile: 47.4% men among those excluded vs. 47.5% of those included, with a mean age of 48.9 and 47.7, respectively. Median BDI-SF score was also the same: 7 points. The median of five of the eight dimensions of SF-36 was similar in both groups. For physical functioning this was 85.0 points for those excluded and 90.0 points for those included, 75.3 and 100.0 for physical and 67.5 and 70.0 for general health, respectively. The prevalence of fatigue was 54.8% vs. 56.9%, SOB 30.5% vs. 22.6%, and LLE 6.3% and 16.9%, respectively. Between June 2006 and December 2007, 13 communities, selected by convenience, were visited and the following evaluations were performed: anthropometric assessment, medical consultation with medical history, family history and physical exam, and a self-administered questionnaire (with support from the researchers) on sociodemographic conditions, lifestyle and mood.
Patients who reported a previous diagnosis of hypertension or those who presented with systolic blood pressure (SBP)≥140mmHg or diastolic blood pressure (DBP)≥90mmHg8 were classified as hypertensive. Patients were classified as diabetic if they had a confirmed previous diagnosis or presented with a fasting glucose level≥126mg/dl.9
The following lipid values were considered elevated: total cholesterol (TC)≥200mg/dl, LDL cholesterol≥130mg/dl, triglycerides (TG)≥150mg/dl, and VLDL cholesterol≥30mg/dl.10 Patients were considered to be hyperuricemic when uric acid was ≥6mg/dl for females and ≥7mg/dl for males. Anemia was defined as hematocrit<40% for males and <36% for females. A body mass index (BMI) of ≥30kg/m2 was defined as obese.11
Statistical analysisThe generalized estimating equation model in the Statistical Package for the Social Sciences (SPSS) version 17.0, which is suitable for non-independent observations (individuals from the same family were selected from the CAMELIA study), was used to calculate crude and adjusted odds ratios (OR). As well as descriptive statistics, crude odds ratios (COR) for SOB, fatigue and LLE were calculated. Adjusted odds ratios (AOR) were estimated, controlling for sociodemographics, lifestyle variables and comorbidities, to measure the association between the dimensions of QOL and HF symptoms. Each of the three symptoms was then considered a risk factor (independent variable) and the eight SF-36 dimensions, one by one, as outcome (dependent) variables. The COR and AOR for a low (below the median score) QOL are shown in Table 2. Table 3 shows the AOR of the QOL dimensions (SF-36) according to the number of symptoms of HF. The statistical significance level was set at 0.05. The combination of symptoms is classified by mean rank in ascending order.
Ethical considerationsThe CAMELIA study was approved on February 3, 2006, by the Ethics Committee of the School of Medicine, Universidade Federal Fluminense/HUAP (CEP CMM/HUAP No. 220/05). All participants signed a consent form allowing their participation in the research and authorizing disclosure of the results obtained in the study.
ResultsThe majority of the 445 participants were between 40 and 49 years of age, female and mulatto, had only attended elementary school and had a per capita family income of up to R$200.00 (the latter two data are not shown). The majority stated the following about their lifestyle-associated cardiovascular risks: they never smoked, had not consumed alcohol regularly over the previous six months, did not use salt at the table and exercised for less than 150minutes per week (they were physically inactive). Only 10% had symptoms of depression. Regarding comorbidities related to cardiovascular risk, the majority were not classified as obese or diabetic but were hypertensive and dyslipidemic. Fatigue was the most prevalent symptom (56.9%), followed by SOB (22.6%) and LLE (16.9%).
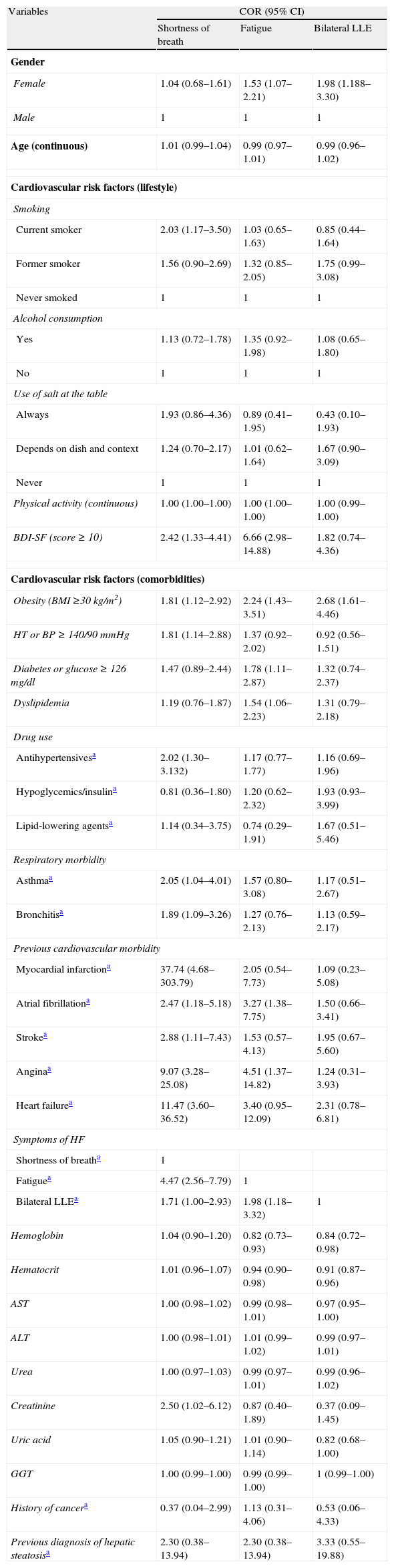
We observed a negative association of breathlessness with race (mulatto) (data not shown) and creatinine and a positive association with smoking, depression (according to the BDI-SF), obesity, hypertension, use of antihypertensives, asthma, bronchitis, myocardial infarction, atrial fibrillation (AF), stroke, angina and HF. Fatigue was negatively associated with hemoglobin and hematocrit and positively with gender (female), depression (according to the BDI-SF), obesity, diabetes, dyslipidemia, atrial fibrillation and angina. A positive association of LLE with female gender and obesity was found. All these variables were entered in the models to control for possible confounding, with the exception of HF. The three symptoms were associated with each other, although the association between SOB and LLE was not statistically significant (Table 1).
Crude odds ratios of symptoms of HF. Niteroi Family Doctor Program, 2006–2008.
| Variables | COR (95% CI) | ||
| Shortness of breath | Fatigue | Bilateral LLE | |
| Gender | |||
| Female | 1.04 (0.68–1.61) | 1.53 (1.07–2.21) | 1.98 (1.188–3.30) |
| Male | 1 | 1 | 1 |
| Age (continuous) | 1.01 (0.99–1.04) | 0.99 (0.97–1.01) | 0.99 (0.96–1.02) |
| Cardiovascular risk factors (lifestyle) | |||
| Smoking | |||
| Current smoker | 2.03 (1.17–3.50) | 1.03 (0.65–1.63) | 0.85 (0.44–1.64) |
| Former smoker | 1.56 (0.90–2.69) | 1.32 (0.85–2.05) | 1.75 (0.99–3.08) |
| Never smoked | 1 | 1 | 1 |
| Alcohol consumption | |||
| Yes | 1.13 (0.72–1.78) | 1.35 (0.92–1.98) | 1.08 (0.65–1.80) |
| No | 1 | 1 | 1 |
| Use of salt at the table | |||
| Always | 1.93 (0.86–4.36) | 0.89 (0.41–1.95) | 0.43 (0.10–1.93) |
| Depends on dish and context | 1.24 (0.70–2.17) | 1.01 (0.62–1.64) | 1.67 (0.90–3.09) |
| Never | 1 | 1 | 1 |
| Physical activity (continuous) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (0.99–1.00) |
| BDI-SF (score≥10) | 2.42 (1.33–4.41) | 6.66 (2.98–14.88) | 1.82 (0.74–4.36) |
| Cardiovascular risk factors (comorbidities) | |||
| Obesity (BMI≥30kg/m2) | 1.81 (1.12–2.92) | 2.24 (1.43–3.51) | 2.68 (1.61–4.46) |
| HT or BP≥140/90mmHg | 1.81 (1.14–2.88) | 1.37 (0.92–2.02) | 0.92 (0.56–1.51) |
| Diabetes or glucose≥126mg/dl | 1.47 (0.89–2.44) | 1.78 (1.11–2.87) | 1.32 (0.74–2.37) |
| Dyslipidemia | 1.19 (0.76–1.87) | 1.54 (1.06–2.23) | 1.31 (0.79–2.18) |
| Drug use | |||
| Antihypertensivesa | 2.02 (1.30–3.132) | 1.17 (0.77–1.77) | 1.16 (0.69–1.96) |
| Hypoglycemics/insulina | 0.81 (0.36–1.80) | 1.20 (0.62–2.32) | 1.93 (0.93–3.99) |
| Lipid-lowering agentsa | 1.14 (0.34–3.75) | 0.74 (0.29–1.91) | 1.67 (0.51–5.46) |
| Respiratory morbidity | |||
| Asthmaa | 2.05 (1.04–4.01) | 1.57 (0.80–3.08) | 1.17 (0.51–2.67) |
| Bronchitisa | 1.89 (1.09–3.26) | 1.27 (0.76–2.13) | 1.13 (0.59–2.17) |
| Previous cardiovascular morbidity | |||
| Myocardial infarctiona | 37.74 (4.68–303.79) | 2.05 (0.54–7.73) | 1.09 (0.23–5.08) |
| Atrial fibrillationa | 2.47 (1.18–5.18) | 3.27 (1.38–7.75) | 1.50 (0.66–3.41) |
| Strokea | 2.88 (1.11–7.43) | 1.53 (0.57–4.13) | 1.95 (0.67–5.60) |
| Anginaa | 9.07 (3.28–25.08) | 4.51 (1.37–14.82) | 1.24 (0.31–3.93) |
| Heart failurea | 11.47 (3.60–36.52) | 3.40 (0.95–12.09) | 2.31 (0.78–6.81) |
| Symptoms of HF | |||
| Shortness of breatha | 1 | ||
| Fatiguea | 4.47 (2.56–7.79) | 1 | |
| Bilateral LLEa | 1.71 (1.00–2.93) | 1.98 (1.18–3.32) | 1 |
| Hemoglobin | 1.04 (0.90–1.20) | 0.82 (0.73–0.93) | 0.84 (0.72–0.98) |
| Hematocrit | 1.01 (0.96–1.07) | 0.94 (0.90–0.98) | 0.91 (0.87–0.96) |
| AST | 1.00 (0.98–1.02) | 0.99 (0.98–1.01) | 0.97 (0.95–1.00) |
| ALT | 1.00 (0.98–1.01) | 1.01 (0.99–1.02) | 0.99 (0.97–1.01) |
| Urea | 1.00 (0.97–1.03) | 0.99 (0.97–1.01) | 0.99 (0.96–1.02) |
| Creatinine | 2.50 (1.02–6.12) | 0.87 (0.40–1.89) | 0.37 (0.09–1.45) |
| Uric acid | 1.05 (0.90–1.21) | 1.01 (0.90–1.14) | 0.82 (0.68–1.00) |
| GGT | 1.00 (0.99–1.00) | 0.99 (0.99–1.00) | 1 (0.99–1.00) |
| History of cancera | 0.37 (0.04–2.99) | 1.13 (0.31–4.06) | 0.53 (0.06–4.33) |
| Previous diagnosis of hepatic steatosisa | 2.30 (0.38–13.94) | 2.30 (0.38–13.94) | 3.33 (0.55–19.88) |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; BDI-SF: Beck Depression Inventory short form; BMI: body mass index; BP: blood pressure; COR: crude odds ratios; GGT: gamma-glutamyl transferase; HF: heart failure; HT: hypertension; LLE: lower limb edema.
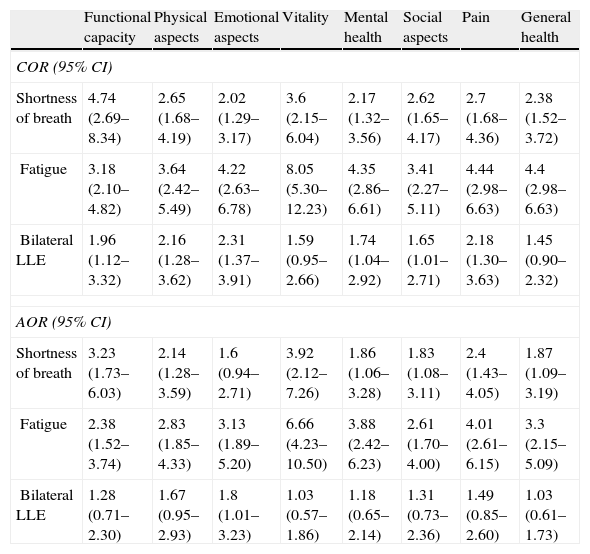
There was a statistically significant crude association between SOB and fatigue with all SF-36 dimensions. The probability of scoring below median on all eight dimensions of the SF-36 was higher for those who reported the presence of these symptoms. The association of LLE with the vitality and general health dimensions did not reach statistical significance. In the adjusted analysis, SOB and fatigue were again associated with all of the SF-36 dimensions, with the exception of the emotional aspects dimension in the case of SOB. LLE was not significantly associated with any of the SF-36 dimensions in the adjusted analysis (Table 2).
Crude and adjusted odds ratios of dimensions of quality of life (SF-36) according to symptoms of heart failure. Niteroi Family Doctor Program, 2006–2008.
| Functional capacity | Physical aspects | Emotional aspects | Vitality | Mental health | Social aspects | Pain | General health | |
| COR (95% CI) | ||||||||
| Shortness of breath | 4.74 (2.69–8.34) | 2.65 (1.68–4.19) | 2.02 (1.29–3.17) | 3.6 (2.15–6.04) | 2.17 (1.32–3.56) | 2.62 (1.65–4.17) | 2.7 (1.68–4.36) | 2.38 (1.52–3.72) |
| Fatigue | 3.18 (2.10–4.82) | 3.64 (2.42–5.49) | 4.22 (2.63–6.78) | 8.05 (5.30–12.23) | 4.35 (2.86–6.61) | 3.41 (2.27–5.11) | 4.44 (2.98–6.63) | 4.4 (2.98–6.63) |
| Bilateral LLE | 1.96 (1.12–3.32) | 2.16 (1.28–3.62) | 2.31 (1.37–3.91) | 1.59 (0.95–2.66) | 1.74 (1.04–2.92) | 1.65 (1.01–2.71) | 2.18 (1.30–3.63) | 1.45 (0.90–2.32) |
| AOR (95% CI) | ||||||||
| Shortness of breath | 3.23 (1.73–6.03) | 2.14 (1.28–3.59) | 1.6 (0.94–2.71) | 3.92 (2.12–7.26) | 1.86 (1.06–3.28) | 1.83 (1.08–3.11) | 2.4 (1.43–4.05) | 1.87 (1.09–3.19) |
| Fatigue | 2.38 (1.52–3.74) | 2.83 (1.85–4.33) | 3.13 (1.89–5.20) | 6.66 (4.23–10.50) | 3.88 (2.42–6.23) | 2.61 (1.70–4.00) | 4.01 (2.61–6.15) | 3.3 (2.15–5.09) |
| Bilateral LLE | 1.28 (0.71–2.30) | 1.67 (0.95–2.93) | 1.8 (1.01–3.23) | 1.03 (0.57–1.86) | 1.18 (0.65–2.14) | 1.31 (0.73–2.36) | 1.49 (0.85–2.60) | 1.03 (0.61–1.73) |
COR: crude odds ratios; AOR: adjusted odds ratios; LLE: lower limb edema.
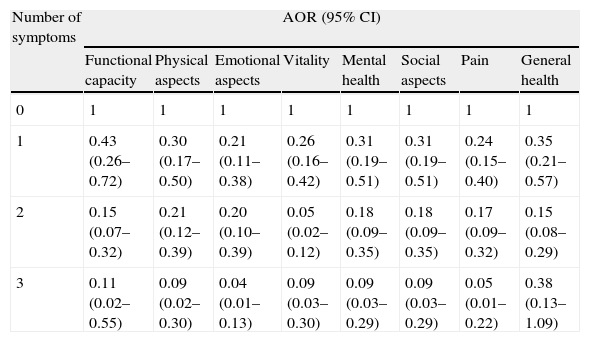
The probability of an above-median QOL score decreased as the number of symptoms increased, independently of gender, age, race, smoking, depression, obesity, diabetes, dyslipidemia, hypertension, antihypertensive medication, asthma/bronchitis, previous myocardial infarction, atrial fibrillation, stroke, angina, creatinine, hemoglobin and hematocrit (Table 3).
Adjusted odds ratios of dimensions of quality of life (SF-36) according to number of symptoms of heart failure. Niteroi Family Doctor Program, 2006–2008.
| Number of symptoms | AOR (95% CI) | |||||||
| Functional capacity | Physical aspects | Emotional aspects | Vitality | Mental health | Social aspects | Pain | General health | |
| 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 0.43 (0.26–0.72) | 0.30 (0.17–0.50) | 0.21 (0.11–0.38) | 0.26 (0.16–0.42) | 0.31 (0.19–0.51) | 0.31 (0.19–0.51) | 0.24 (0.15–0.40) | 0.35 (0.21–0.57) |
| 2 | 0.15 (0.07–0.32) | 0.21 (0.12–0.39) | 0.20 (0.10–0.39) | 0.05 (0.02–0.12) | 0.18 (0.09–0.35) | 0.18 (0.09–0.35) | 0.17 (0.09–0.32) | 0.15 (0.08–0.29) |
| 3 | 0.11 (0.02–0.55) | 0.09 (0.02–0.30) | 0.04 (0.01–0.13) | 0.09 (0.03–0.30) | 0.09 (0.03–0.29) | 0.09 (0.03–0.29) | 0.05 (0.01–0.22) | 0.38 (0.13–1.09) |
AOR: adjusted odds ratios. Adjusted for gender, age, race, smoking, depression, obesity, diabetes, dyslipidemia, hypertension, antihypertensive medication, asthma/bronchitis, myocardial infarction, atrial fibrillation, stroke, angina, creatinine, hemoglobin and hematocrit.
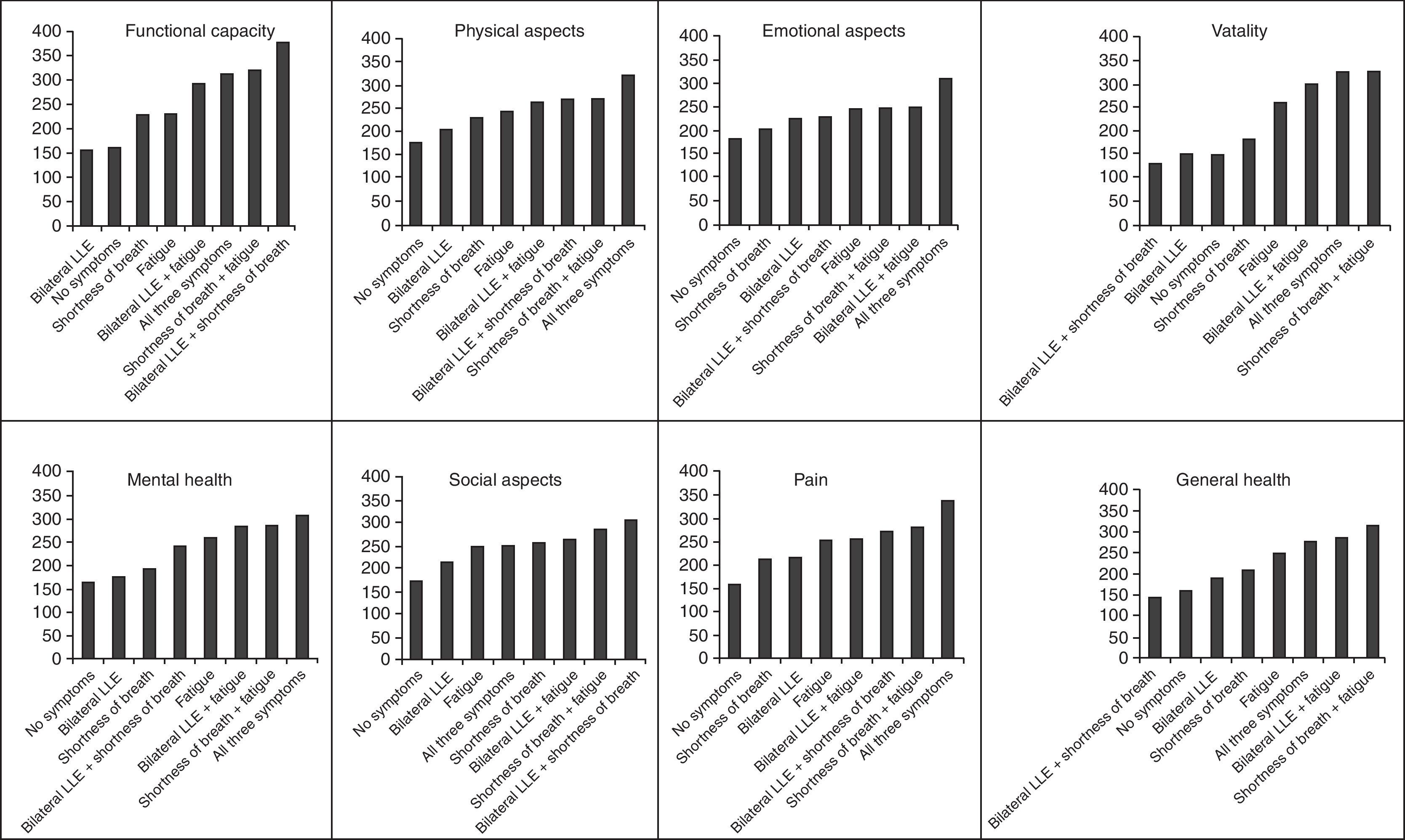
In five of the eight dimensions (functional capacity, physical aspects, emotional aspects, mental health and pain), an increase in the number of symptoms was directly associated with an increased probability of a below-median score on the QOL questionnaire. For the vitality, social aspects and general health dimensions, the probability of a below-median score increased for up to two symptoms and decreased when a third symptom was present (data not shown). Combinations of the three symptoms did not show the same pattern of impact on QOL for all of the dimensions. On most dimensions, LLE and SOB had less impact on QOL than fatigue (Figure 1).
DiscussionMain finding of the studyIn this study of patients receiving PC, the HF symptoms SOB and fatigue were found to be independently associated with all of the QOL dimensions, except for the emotional aspects dimension in the case of SOB. In contrast, LLE displayed no statistically significant associations with any of the SF-36 dimensions.
In the scenario addressed by this study, access to technologically intensive methods for confirming an HF diagnosis is limited due to cost and the limited availability of skilled professionals.4 In this study, a confirmed diagnosis of HF was not available due to lack of access to standard recommended tests.
What is already known on this topicFatigue (also described as tiredness), SOB and LLE are the most common symptoms of HF, with SOB being the most frequent.3 All three symptoms are intermittent and can affect healthy people as well as those affected by other medical conditions not necessarily related to heart disease;12 they therefore have low specificity for HF diagnosis.
In this study, the most common symptom was fatigue (57%), followed by SOB (23%) and LLE (17%). McIlvenny et al.,13 in a study of 254 individuals conducted in the United Arab Emirates, found a prevalence of fatigue of 34% in males and 38% in females, based on responses to a questionnaire which included scales of fatigue.
Population studies have found a prevalence of SOB ranging from 8.9% in the Australian population14 to 12.6% in the Swedish population.15 Ahmed et al.,16 in an evaluation of the medical records of 1007 patients treated in a PC setting, found a prevalence of LLE of 9.4%. Studies have indicated a high prevalence of these symptoms in patients with chronic HF. Prevalences of SOB and fatigue vary from 54% and 53%, respectively, in HF patients aged over 60 years in the community17 to 90% and 73%, respectively, in hospitalized patients with chronic New York Heart Association (NYHA) class III and IV HF.18 LLE varied from 17% in elderly patients with HF17 to 66% in hospitalized patients with chronic NYHA class III and IV HF.18
The prevalences observed in the present study were higher than those found in the general population and lower than those observed in patients with HF, which can be explained by the basic study design predominantly including females with higher prevalences of hypertension and diabetes than in the general population.
Health-related quality of life (HRQL) is increasingly used as an outcome in clinical trials,19 with studies on HRQL most frequently involving cancer and other chronic diseases, such as HF. In our study, the sum of all symptoms was significantly related to all the QOL dimensions, as shown in Figure 1.
HF is often an insidious syndrome, and its diagnosis in a PC setting is hampered by limited access to standard diagnostic methods. Symptoms alone are not good predictors of HF. On one hand, they may show low specificity, since symptoms may be affected by both objective and subjective factors that are not specific to HF.20 On the other hand, sensitivity is also low because, in many cases, HF diagnosis is not preceded by symptoms (patient complaints) or by signs on physical examination by a physician.4 It has been shown that the burden of functional capacity (physical appearance and physical capacity) in patients with HF is significantly higher than in those suffering from other serious chronic diseases, whether cardiac or involving other systems.21
Although the Third Brazilian Guidelines for Chronic Heart Failure (2009) recommend a therapeutic approach to HF when the symptoms examined in this study are present, this recommendation is rarely followed, and most patients are only diagnosed and treated at advanced stages of the disease.
What this study addsThe association of patient complaints, particularly SOB and fatigue (in the absence of other conditions that may be responsible for those symptoms), with QOL assessment can decrease the percentage of false positives, thereby increasing the positive predictive value for clinical diagnosis. This increase may enable increased accuracy in patient triage and in referral of patients for more expensive diagnostic procedures, thereby shortening the time between diagnosis and treatment and improving patient prognosis.
The findings of this study also suggest directions for further investigation. Studies testing whether the combination of SOB and fatigue, along with low QOL scores in the absence of other health conditions that can explain the symptoms, is a marker for HF, could help to determine the utility of this patient presentation in prioritizing access to imaging and laboratory tests.
Study limitationsThis study has some limitations. Of the total population, 31.48% participants were excluded from the analysis due to lack of complete clinical data. However, the losses were considered random because they were mostly due to technical difficulties. The comparison between samples of included and excluded individuals showed a similar demographic profile and median scores on the BDI-SF and five of the eight dimensions of the SF-36. However, the excluded group presented a higher prevalence of SOB and LLE, but not of fatigue. Another limitation was the failure to adjust the association between symptoms and QOL for thyroid disease, since thyroid hormones were not measured. That is, although we adjusted the association between symptoms and QOL for other possible causes of symptoms, we did not do so for thyroid disease, which may be an additional underlying cause to HF of the association studied.
ConclusionsThe prevalence of SOB was 22.6%, fatigue 56.9% and LLE 16.9%. These results are intermediate between those of general populations and those of patients with HF.
The associations with the dimensions of the SF-36 were statistically significant, independent of demographic and socioeconomic factors, metabolic characteristics and comorbidities, for SOB and fatigue but not for LLE.
Neither symptoms nor QOL have good predictive accuracy for a diagnosis of HF. The combination of symptoms (especially SOB and fatigue) with QOL has increased positive predictive value for an HF diagnosis and may be used as part of the triage process.
Conflict of interestThe authors have no conflict of interest to declare.