No evidence-based therapy has yet been established for Takotsubo syndrome (TTS). Given the putative harmful effects of catecholamines in patients with TTS, beta-blockers may potentially decrease the intensity of the detrimental cardiac effects in those patients.
ObjectiveThe purpose of this study was to assess the impact of beta-blocker therapy on long-term mortality and TTS recurrence.
MethodsThe cohort study used the national Spanish Registry on TakoTsubo Syndrome (RETAKO). A total of 970 TTS post-discharge survivors, without pheochromocytoma, left ventricular outflow tract obstruction, sustained ventricular arrhythmias, and significant bradyarrhythmias, between January 1, 2003, and July 31, 2018, were assessed. Cox regression analysis and inverse probability weighting (IPW) propensity score analysis were used to evaluate the association between beta-blocker therapy and survival free of TTS recurrence.
ResultsFrom 970 TTS patients, 582 (60.0%) received beta-blockers. During a mean follow-up of 2.5±3.3 years, there were 87 deaths (3.6 per 100 patients/year) and 29 TTS recurrences (1.2 per 100 patient/year). There was no significant difference in follow-up mortality or TTS recurrence in unadjusted and adjusted Cox analysis (hazard ratio [HR] 0.86, 95% confidence interval [CI] 0.59–1.27, and 0.95, 95% CI 0.57–1.13, respectively). After weighting and adjusting by IPW, differences in one-year survival free of TTS recurrence between patients treated and untreated with beta-blockers were not found (average treatment effect −0.01, 95% CI −0.07 to 0.04; p=0.621).
ConclusionsIn this observational nationwide study from Spain, there was no significant association between beta-blocker therapy and follow-up survival free of TTS recurrence.
Ainda não foi estabelecido um tratamento baseado na evidência para a síndrome de Takotsubo (STT). Dados os efeitos supostamente nocivos das catecolaminas em doentes com STT, os betabloqueantes podem potencialmente diminuir a intensidade dos seus efeitos prejudiciais nesses doentes.
ObjetivoAvaliar o impacto da terapêutica com betabloqueantes na mortalidade a longo prazo e na recidiva da STT.
MétodosO estudo coorte utilizou o registo nacional espanhol da síndrome de Takotsubo (RETAKO). Foram avaliados 970 sobreviventes de STT após a alta, sem feocromocitoma, sem obstrução do trato de saída do ventrículo esquerdo, sem arritmias ventriculares mantidas e sem bradiarritmias significativas, entre 1 de janeiro de 2003 e 31 de julho de 2018. A análise de regressão de Cox e a análise da ponderação de probabilidade inversa (PPI) foram utilizadas para avaliar a associação entre a terapia com betabloqueantes e a sobrevivência sem recidiva de STT.
ResultadosDos 970 doentes com STT, 582 (60,0%) foram tratados com betabloqueantes. Durante um seguimento médio de 2,5±3,3 anos, houve 87 mortes (3,6 por 100 doentes/ano) e 29 recidivas de STT (1,2 por 100 doentes/ano). Não se verificaram diferenças significativas na mortalidade durante o seguimento, nem na recidiva de STT na análise Cox não ajustada e ajustada (Hazard Ratio [HR] 0,86, Intervalo de Confiança [IC] 95%, 0,59-1,27 e IC 95%−0,57-1,13, respetivamente). Após ponderação e ajuste por PPI, não foram também encontradas diferenças na sobrevivência a um ano e na recidiva de STT entre doentes tratados e não tratados com betabloqueantes (efeito médio do tratamento −0,01, IC 95% −0,07 a 0,04; p=0,621).
ConclusãoNeste estudo observacional de âmbito nacional em Espanha, não houve associação significativa entre a terapêutica com betabloqueantes e a sobrevivência ou recidivas de STT.
Beta-blockers are one of the medical therapies most frequently prescribed to patients with Takotsubo syndrome (TTS) at discharge.1 Taking into account its physiopathology, it seems logical to use beta-blockers in order to protect against stressful triggers and subsequent catecholamine surges.2 However, clinical uncertainty exists regarding their effectiveness in reducing mortality in TTS patients, since there is no evidence of any survival benefit of using beta-blockers after hospital discharge.3,4
Moreover, post-discharge beta-blocker therapy does not appear to prevent recurrence. Up to a third of patients experienced a TTS recurrence during treatment with beta-blockers, suggesting that other receptors such as alpha-receptors might be involved.5–7 The International Expert Consensus Document on Takotsubo Syndrome suggests that beta-blockers might be useful for preventing recurrence of TTS in selected patients, especially those with persistent anxiety and elevated sympathetic tone.8
To the best of our knowledge, to date there are no analyses of large-scale datasets that have specifically investigated the impact of beta-blockers on survival after hospital discharge for TTS. The aim of this study is to fill this gap by assessing the long-term prognostic impact of beta-blockers in TTS patients according to the pattern and trigger.
MethodsStudy populationThe analyses were based on data from the national multicenter RETAKO (Registry on Takotsubo Syndrome) trial, supported by the Ischemic Heart Disease and Acute Cardiovascular Care Section of the Spanish Society of Cardiology. It is a partially retrospective and prospective (from January 1, 2012, onwards), voluntary, observational study that enrolled TTS patients from 38 centers in Spain. Data were collected at each hospital, electronically encrypted and anonymized, and transferred online to a central database. Its rationale and design have been previously described.9,10
The main inclusion criteria required a definitive TTS diagnosis based on the modified Mayo Clinic criteria11: (1) transient left ventricular dysfunction with apical, midventricular, or basal segmental alterations extending beyond the territory supplied by a single coronary artery; (2) angiographic absence of significant obstructive coronary disease (luminal narrowing >50%) or a complicated (ruptured/thrombosed) atheroma; (3) new electrocardiographic changes (ST-segment elevation and/or negative T waves) and moderate elevation of cardiac troponins. Complete normalization of wall motion abnormalities and left ventricular ejection fraction (LVEF) was required, except in cases that died before complete normalization of LVEF.
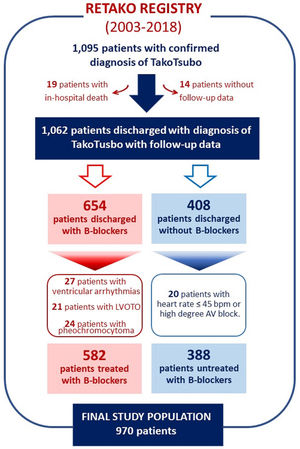
Our cohort (N=970) was obtained from 1095 patients with final diagnosis of TTS admitted between January 1, 2003, and July 31, 2018 (see Figure 1). We excluded 19 (1.7%) patients who died in the hospital; 14 (1.3%) with missing mortality data; 24 (2.2%) with pheochromocytoma; 21 (1.9%) with left ventricular outflow tract obstruction (LVOTO), defined as a resting peak instantaneous gradient >25 mmHg in absence of inotropics; 27 (2.5%) with sustained ventricular arrhythmias; and 20 (1.8%) with baseline heart rate ≤45 bpm or high degree atrial-ventricular heart block. For the present study, beta-blocker use was determined according to whether eligible patients had received beta-blockers on discharge from the hospital.
The study complied with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Hospital Clínico San Carlos (Madrid, Spain). All patients provided written informed consent.
Triggers and pattern of takotsubo syndromeAll the possible triggers in each patient were collected. Patients were categorized into groups according to the types of preceding stressors – emotional stress, physical stress, and no identifiable trigger – based on the recently proposed classification.12 The pattern of LV dysfunction based on angiography or echocardiographic evaluation was classified as apical and non-apical types.8 Apical type is characterized by hypokinesia, akinesia or dyskinesia of midventricular and apical parts of the anterior, septal, inferior and lateral walls of the left ventricle, associated with hyperkinesia of basal segments. Non-apical pattern includes all the remaining types (basal, midventricular, and focal types).
Follow-up and outcomesFollow-up after hospital discharge was conducted either by outpatient clinical visits or structured telephone interviews with patients or relatives. Follow-up outcomes were pre-specified and defined as the first non-fatal TTS recurrence or the occurrence of all-cause death. Recurrence was considered if a documented new non-fatal episode met the diagnostic criteria for TTS.11 If a pre-specified outcome was observed, review of electronic medical records and consensus of two experienced local investigators were mandatory for event adjudication. Patients were censored if they did not experience the outcome of interest or were lost to follow-up.
Statistical analysisBaseline characteristics according to treatment with beta-blockers were described by using number and percentage for categorical data and mean±standard difference for continuous data. Differences in characteristics were assessed by using Chi-square tests and two-sample Student t-tests, for categorial and continuous variables, respectively.
The association between beta-blocker therapy and survival free of TTS recurrence was studied in robust Cox regression analysis. Multivariable model risk adjustments were performed with all variables associated with the combined outcome of post-discharge mortality or TTS recurrence based on clinical plausibility or p-value <0.1 in the univariate Cox analyses (Table 1 of the supplementary data). Estimates were expressed as hazard ratios (HRs) with their 95% confidence interval (CI). Proportionality of risk assumption of survival models was evaluated by plotting Schoenfeld residuals or by studying the interaction of covariates with time for the Cox proportional hazard models.
Given the substantial differences in key baseline characteristics depending on prescription of beta-blocker therapy, we performed a sensitivity analysis using a propensity score (PS) analysis. PSs were estimated using a non-parsimonious multivariable logistic regression model, with beta-blocker therapy as the dependent variable and those characteristics that differed between patients treated and not treated with beta-blockers (Table 1) as covariates. Survival time inverse probability weighting (IPW) PS analysis was used to evaluate the association between beta-blocker therapy and survival free of TTS recurrence. The effect of beta-blockers was graphically represented in Kaplan-Meier curves adjusted by IPW, to balance the covariate distribution between the treatment and control observations.13 We complemented the analysis with an augmented IPW (AIPW) to estimate the average treatment effect (ATE) of beta-blockers in one-year survival free of TTS recurrence, using a doubly robust method that combined both the properties of the regression-based estimator and the IPW estimator.14 Subsequent PS matching (PSM) was performed to assemble a cohort in which all the measured covariates would be well balanced across the comparator group. PSM was performed with 1:1 nearest-neighbor matching without replacement and with a caliper width of 0.2 of the standard deviation of all PSs. Standard mean differences were estimated for all covariates before and after matching to assess pre-matching imbalance and post-matching balance; standard mean differences of <10% for a given covariate were considered as indicators of adequate balance (Table 2). In the PS-matched population, the reduction in mortality and/or TTS recurrence was compared using a stratified Cox regression model.
Baseline characteristics according to the prescription of beta-blockers.
| Variables | Total population(n=970) | beta-Blockers(n=582, 60.0%) | Non beta-blockers(n=388, 40.0%) | p-Value |
|---|---|---|---|---|
| Age | 70.8±11.7 | 70.5±11.4 | 71.3±12.0 | 0.319 |
| Female sex | 854 (88.0%) | 523 (89.9%) | 331 (85.3%) | 0.032 |
| Cardiovascular risk factors | ||||
| Smoking | 125 (12.9%) | 66 (11.3%) | 59 (15.2%) | 0.078 |
| Hypertension | 628 (64.7%) | 393 (67.5%) | 235 (60.6%) | 0.026 |
| Dyslipemia | 444 (45.8%) | 272 (46.7%) | 172 (44.3%) | 0.461 |
| Diabetes mellitus | 184 (19.0%) | 112 (19.2%) | 72 (18.6%) | 0.789 |
| Cardiovascular history | ||||
| Peripheral artery disease | 32 (3.3%) | 22 (3.8%) | 10 (2.6%) | 0.304 |
| Coronary artery disease | 70 (7.2%) | 40 (6.9%) | 30 (7.7%) | 0.612 |
| Prior stroke | 70 (7.2%) | 50 (8.6%) | 20 (5.2%) | 0.043 |
| Prior beta-blocker therapy | 91 (9.4%) | 72 (12.4%) | 19 (4.9%) | <0.001 |
| Comorbidities | ||||
| COPD | 149 (15.4%) | 69 (11.9%) | 80 (20.6%) | <0.001 |
| History of cancer | 108 (11.1%) | 54 (9.3%) | 54 (13.9%) | 0.024 |
| Presenting characteristics | ||||
| Killip≥II at admission | 318 (32.8%) | 176 (30.2%) | 142 (36.6%) | 0.039 |
| Atrial fibrillation/flutter | 172 (17.7%) | 104 (17.9%) | 68 (17.5%) | 0.891 |
| Bundle brunch block | 54 (5.6%) | 38 (6.5%) | 16 (4.1%) | 0.109 |
| Creatinine >1.5 mg/dl | 47 (4.8%) | 35 (6.0%) | 12 (3.1%) | 0.038 |
| Treatment at discharge | ||||
| Aspirin | 454 (46.8%) | 329 (56.5%) | 125 (32.2%) | <0.001 |
| P2Y12 inhibitor | 67 (6.9%) | 49 (8.4%) | 18 (4.6%) | 0.023 |
| Oral anticoagulation | 166 (17.1%) | 117 (20.1%) | 49 (12.6%) | 0.002 |
| ACEI/ARB | 594 (61.2%) | 426 (73.2%) | 168 (43.3%) | <0.001 |
| Calcium channel blockers | 85 (8.8%) | 38 (6.5%) | 47 (12.1%) | 0.003 |
| Nitrates | 35 (3.6%) | 21 (3.6%) | 14 (3.6%) | 1.00 |
| Diuretics | 227 (23.4%) | 147 (25.3%) | 80 (20.6%) | 0.095 |
| Statins | 477 (49.2%) | 345 (59.3%) | 132 (34.0%) | <0.001 |
| Anxiolytic medication | 268 (27.6%) | 187 (32.1%) | 81 (20.9%) | <0.001 |
| Antidepressant therapy | 130 (13.4%) | 90 (15.5%) | 40 (10.3%) | 0.021 |
Values are mean±standard difference or n (%).
ACEI/ARB: angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers; COPD: chronic obstructive pulmonary disease.
Average treatment effect (ATE) of beta-blockers in mortality and recurrence of Takotsubo (TTS) after weighting and adjustment for baseline characteristics.
| TTS population and subgroups | Average treatment effect | ||
|---|---|---|---|
| Coefficient | 95% confidence interval | p-Value | |
| Total TTS | −0.01 | −0.07 to 0.04 | 0.621 |
| TTS type by location | |||
| Apical TTS | −0.04 | −0.11 to 0.03 | 0.292 |
| Non-apical TTS | 0.06 | −0.04 to 0.15 | 0.256 |
| Classification by trigger | |||
| Emotional stress | 0.01 | −0.10 to 0.12 | 0.871 |
| Physical stress | 0.03 | −0.06 to 0.11 | 0.517 |
| Non identifiable trigger | −0.08 | −0.19 to 0.03 | 0.132 |
ATE: average treatment effect; TTS: Takotsubo syndrome.
The average treatment effects (ATEs) represent the absolute difference in mortality or TTS recurrence at one year between beta-blockers treatment versus no treatment across the whole cohort (comparing mortality and TTS recurrence in a scenario in which all patients were treated versus mortality and TTS recurrence in a scenario in which no patients were treated).
Analysis of follow-up mortality and TTS recurrence was also performed in subgroups defined according to age (<75 years or ≥75 years), sex, presence of coronary artery disease, atrial fibrillation/flutter, chronic obstructive pulmonary disease (COPD), Killip class, status of cardiogenic shock, and therapy with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEI/ARB) on discharge. Treatment-by-subgroup interactions were tested using a Cox proportional hazards model adjusted by IPW, containing the beta-blocker therapy, subgroup, and the treatment-by-subgroup interaction.
A two-tailed p<0.05 indicated statistical significance. Statistical analyses were performed using Stata 15.1 (StataCorp, College Station, Texas).
ResultsComparison of Takotsubo patients treated and untreated with beta-blockersAmong the 970 patients with TTS included in this study, 582 patients (60.0%) were treated with beta-blockers. There were significant differences in baseline characteristics between patients with and without beta-blocker treatment (Table 1). In particular, patients who did not receive beta-blockers were more frequently comorbid, including COPD (20.6% vs. 11.9%) and history of cancer (13.9% vs. 9.3%). Compared with patients untreated with beta-blockers, those who received beta-blockers tended to be treated with higher rates of aspirin (56.5% vs. 32.2%; p<0.001), ACEI/ARB (73.2% vs. 43.3%; p<0.001), and statins (59.3% vs. 34.0%; p<0.001).
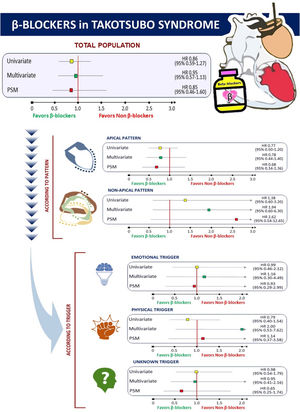
Prognosis according to beta-blocker therapyFor the entire cohort, during a mean follow-up of 2.5±3.3 years, there were 87 deaths (3.6 per 100 patients/year) and 29 TTS recurrences (1.2 per 100 patients/year). Unadjusted mortality rates were similar for patients who received beta-blockers compared with those who did not [3.3 (95% CI 2.4–4.3) vs. 4.2 (95% CI 3.1–5.7) per 100 patients/year, respectively; p=0.254)]. A similar result was found for TTS recurrence [1.4 (95% CI 0.9–2.1) vs. 0.9 (95% CI 0.4–1.8) per 100 patients/year, respectively; p=0.278)]. After multivariate adjustment (including age, sex, hypertension, diabetes mellitus, smoking, coronary artery disease, prior stroke, prior beta-blocker therapy, COPD, history of cancer, Killip≥II on admission, atrial fibrillation/flutter, creatinine ≥1.5 mg/dl, apical vs. non-apical TTS, emotional or physical trigger, and medication at discharge – aspirin, P2Y12 inhibitors, oral anticoagulation, ACEI/ARB, calcium channel blockers, diuretics, statins, anxiolytics, and antidepressants), there were no significant differences in the composite outcome of mortality or TTS recurrence between patients treated and untreated with beta-blockers (adjusted HR 0.95, 95% CI 0.57–1.53; p=0.794).
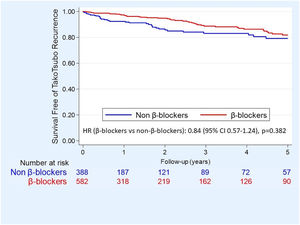
Propensity score analysisFor the balanced PS analysis, 566 patients were removed (380 from the beta-blocker group and 186 from the non-beta-blocker group), leaving 404 patients (202 in each group). Overlap assumption assessment and balance checks were conducted and the results are summarized in Table 2 of the supplementary data. The area under the curve for the PS model was 0.87 (95% CI 0.85–0.89), which indicated a good discrimination for the model. Beta-blocker therapy was not associated with significant differences in dmortality or TTS recurrence neither after adjusting by IPW (Figure 2) nor after PS matching (HR 0.85, 95% CI 0.46–1.60, p=0.621). After weighting and adjustment, differences in one-year survival free of TTS recurrence between patients treated and untreated with beta-blockers were not found (average treatment effect (ATE) 95% −0.01 95% CI −0.07 to 0.04; p=0.621) (Table 2).
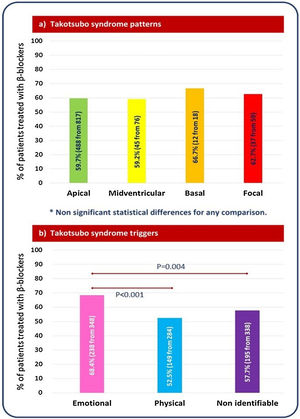
Impact of beta-blocker therapy according to Takotsubo syndrome type and triggerBeta-blocker therapy was similarly prescribed in the four major TTS types (Figure 3A). Lack of benefit of beta-blockers in terms of follow-up mortality and/or TTS recurrence was consistent across different TTS types (Figure 4: central illustration). In the PS-matched population, the HR for mortality or TTS recurrence of beta-blockers in patients with apical TTS was 0.68 (95% CI 0.34–1.36, p-value=0.277), and in patients with non-apical TTS was 2.62 (95% CI 0.54–12.65, p-value=0.231). Regarding to the trigger, beta-blocker therapy was most frequently prescribed in patients with TTS related to emotional stress (68.4% vs. 52.5% in TTS related to physical stress, and 57.7% in TTS without an identifiable triggering factor) (Figure 3B). However, no differences in mortality and TTS recurrence were found between patients treated and untreated with beta-blockers, neither after PSM (Figure 4: central illustration) nor after IPW (Table 2).
Subgroup analysisSubgroup analysis after adjusting by IPW has not found a benefit of beta-blockers in any group (Table 3). Even in patients with COPD, beta-blockers were associated with higher rates of mortality or TTS recurrence (HR 2.42, 95% CI 1.07–5.49; p=0.034) (Table 3).
Subgroup analyses of follow-up mortality and Takotsubo recurrence after adjusting by inverse probability weighting (IPW) propensity score analysis.
| Subgroup | HR | 95% CI | p | P for interaction |
|---|---|---|---|---|
| Age | ||||
| <75 years | 1.11 | 0.55–2.21 | 0.773 | 0.594 |
| ≥75 years | 0.84 | 0.52–1.37 | 0.423 | |
| Gender | ||||
| Female | 1.94 | 0.51–7.44 | 0.334 | 0.136 |
| Male | 0.75 | 0.50–1.14 | 0.179 | |
| Coronary artery disease | ||||
| Yes | 1.64 | 0.48–5.67 | 0.433 | 0.339 |
| No | 0.80 | 0.53–1.21 | 0.293 | |
| Atrial fibrillation/flutter | ||||
| Yes | 0.65 | 0.29–1.45 | 0.294 | 0.628 |
| No | 0.88 | 0.56–1.38 | 0.568 | |
| COPD | ||||
| Yes | 0.75 | 0.47–1.18 | 0.212 | 0.004 |
| No | 2.42 | 1.07–5.49 | 0.034 | |
| Killip class | ||||
| I | 0.94 | 0.53–1.64 | 0.823 | 0.644 |
| ≥II | 0.77 | 0.45–1.32 | 0.345 | |
| Cardiogenic shock | ||||
| Yes | 0.31 | 0.10–1.59 | 0.162 | 0.163 |
| No | 0.93 | 0.62–1.40 | 0.731 | |
| ACEI/ARB | ||||
| Yes | 1.02 | 0.55–1.90 | 0.945 | 0.866 |
| No | 0.93 | 0.53–1.62 | 0.794 | |
ACEI/ARB: angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers; CI: confidence interval; COPD: chronic obstructive pulmonary disease; HR: hazard ratio.
In this observational nationwide cohort study of TTS survivors, the use of beta-blockers was not associated with mortality or TTS recurrence.
A major role of excessive adrenergic overstimulation leading to myocardial stunning has been advocated as possible explanation of the pathophysiology of TTS.2 In this context, beta-blockers could potentially reduce the myocardial damage caused by a sympathetic drive. However, our results have not confirmed this hypothesis. Moreover, our lack of association between beta-blockers in TTS and poor outcomes are consistent with other previous studies. In this sense, Isogai et al. examined whether early beta-blocker therapy in patients with TTS was associated with lower in-hospital mortality.3 Using PSM and instrumental variable analyses in 2672 TTS patients, they did not find significant association between early beta-blocker use and in-hospital mortality (odds ratio (OR) 1.08, 95% CI 0.59–1.96, p=0.812). In addition to the results of the Japanese TTS registry, in the InterTAK study there was no evidence of any survival benefit at one year for the use of beta-blockers.4 Moreover, of 57 patients with recurrent TTS in the InterTAK, 29 were taking beta-blockers at the time of the second event. And two meta-analyses were consistent with the neutral effect of beta-blockers in survival free of TTS recurrence. In the meta-analysis of Singh et al., with 847 patients, there was no significant association between the rate of TTS recurrence and use of beta-blockers (p=0.280).7 Similarly, in the meta-analysis of clinical non-randomized registries conducted by Santoro et al., with 471 subjects, the recurrence rate was 1.81% (6 of 331) in those treated with beta-blockers, compared with 2.86% (4 of 140) in controls (OR: 0.44, 95% CI: 0.15–1.31, p=0.790).6 All these data suggest that beta-blockers are not effective in preventing TTS.
One possible pathophysiological explanation justifying our findings might be that the potential protective effect of beta-blockers might be hampered by some paradox phenomena. Paur et al. have proved that beta-blockers that activate beta2 adrenoceptor (AR) to inhibitory G (Gi) protein signaling (beta2AR-Gi) might exacerbate the epinephrine-induced negative inotropic effect.15 High doses of epinephrine can act as a negative inotrope via ligand-mediated trafficking of the beta2-AR from stimulatory G (Gs) protein to Gi protein subcellular signaling pathways, a process described as biased agonism.16 This switch would be favored in conditions of high catecholamine stress because it depends on beta2-AR phosphorylation by both protein kinase A (PKA) and G-protein receptor-coupled kinases (GRKs).17 This is particularly relevant given the increased frequency of the L41Q GRK5 polymorphism, known to increase cardiac GRK5 activity and beta-AR phosphorylation, in a recent study that genotyped patients with Takotsubo cardiomyopathy.18 The Gi-dependent negative effect of beta-blockers was most readily seen in myocytes from failing human hearts (in which Gi is increased), but the group of Paur et al. also analyzed it in TTS patients.15 They evaluated the response of a TTS animal model induced by epinephrine bolus infusion to a propranolol, carvedilol and bisoprolol. Propranolol, a beta-blocker with higher beta2-AR-Gi agonism, enhanced the negative effects of epinephrine at both apex and base, whereas carvedilol, with lower beta2-AR-Gi agonism, had little effect on the apex but decreased the base contraction. In contrast, the beta1-AR selective blocker bisoprolol reduced the positive effect of epinephrine at the base but without effect on the apical epinephrine response. Therefore, drugs with high beta2-AR-Gi agonism (e.g., propranolol, carvedilol) should be avoided in TTS patients. In this sense, there are very few studies that evaluated the impact of the type of beta-blocker on clinical outcomes in this context. A retrospective study by Palla et al. found that, in 64 patients with TTS, of which 16 were taking a beta-blocker on presentation (metoprolol succinate [50%], metoprolol tartrate [37.5%], or atenolol [12.5%]), pretreatment with low dose beta-adrenergic antagonist therapy does not affect severity of TTS presentation.19 Another important issue that remains to be elucidated is the role of beta-blockers dose. The adrenergic blockade achieved with commonly used doses of beta-adrenergic antagonists may not be sufficient to effectively antagonize the supraphysiologic. Unfortunately, we lack information about the type and dose of beta-blocker therapy.
In our subgroup analysis, there was a significant interaction between beta-blocker therapy and COPD on follow-up mortality or TTS recurrence. In COPD patients with TTS, beta-blockers were associated with higher rates of mortality or TTS recurrence, whereas in non-COPD patients, survival free of TTS recurrence was similar in patients treated and untreated with beta-blockers. Although this result should be interpreted cautiously because of the number of patients and the observational – non-randomized – nature of our study, these findings are consistent with the results from the prospective, randomized, BLOCK COPD trial, that showed metoprolol treatment was associated with almost double the risk of experiencing an exacerbation leading to hospitalization (HR 1.91, 95% CI 1.29–2.83) in COPD patients without cardiovascular disease.20
Regarding cardiogenic shock, we have not found an interaction or benefit of beta-blockers in TTS patient with or without cardiogenic shock. Almendro-Delia et al. have previously found a possible association between beta-blockers and a better long-term prognosis of TTS patients.9 Individuals prone to developing hemodynamic instability and cardiogenic shock in the acute phase of TTS may constitute a high-risk phenotype in which predisposing factors could converge: (1) an exaggerated sympathetic activation in response to triggering events; (2) greater susceptibility to catecholamine mediated myocardial injury; or (3) the presence of a decreased cardiac reserve masking a long-term heart failure phenotype as a marker for underlying disease severity.9 The preceding features could lead to a potential long-term prognostic benefit of beta-blockers in TTS patients who present with cardiogenic shock. However, our results, after excluding patients with LVOTO and ventricular arrhythmias, have not confirmed those hypotheses.
To date neither the short- nor long-term prognostic impact of beta-blockers according to the type and trigger of TTS has been proven.21 Previous studies have suggested the existence of different phenotypes within the clinical spectrum of TTS, with different prognosis.4,12 In our study, we have assessed the prognosis of beta-blocker therapy in patients with apical versus non-apical TTS. It has been recently shown that the apical ventricular region has a greater beta2:beta1 adrenoceptor ratio, with a higher responsiveness and vulnerability to sympathetic stimulation.22 The different occurrence of wall motion abnormalities could be explained by interindividual anatomical differences in the distribution of beta-adrenergic receptors. Although in our study treatment with beta-blockers was associated with better prognosis in patients with apical TTS and worse prognosis in those with non-apical TTS, the differences were not significant. Regarding the trigger, we have not found a benefit of beta-blockers in any of the three groups (emotional stress, physical stress, and no identifiable trigger).
Finally, although the present study did not investigate the underlying pathophysiology of TTS, our results regarding beta-blocker therapy might suggest that other pathophysiological pathways such as an inflammatory23 or energetic-metabolic pathway24 need to be explored to identify a suitable therapy for TTS.
Study limitationsOur results should be interpreted in the context of several limitations. First, it is possible that various subclasses, doses and durations of beta-blocker therapy were used in this study. This heterogeneity of beta-blocker therapy might have affected the outcomes. However, those data were not collected in our registry. In addition, we have no data about rates of discontinuation or new prescriptions during follow-up. Second, because of its observational nature, unmeasured confounders constrain causal inference in the present study. Although propensity scoring and further adjustments were made for many additional confounders in the survival models, residual confounding is likely to happen anyway. So cautious interpretation is encouraged and, therefore, prognostic associations should be considered exploratory. Nonetheless, our results are consistent with other non-randomized data. Clearly, a randomized controlled trial is necessary to further investigate the issues surrounding beta-blocker use in TTS.
ConclusionsAmong TTS patients who survived hospitalization, beta-blocker therapy was not associated with lower mortality and TTS recurrence after addressing potential confounding by adjusting and using PS methods. This result adds to the increasing body of evidence that the routine prescription of beta-blockers might not be indicated in patients with TTS.
FundingThe Retako webpage was funded by a non-conditional Astrazeneca scholarship.
Conflict of interestNone declared.